��Ŀ����
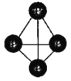
����Ŀ��ֱ�Ӽ״�ȼ�ϵ�� (DMFC) �߱����¿���������ȼ�Ͻྻ�����Լ���ؽṹ�����ԡ���ʹ��ֱ�Ӽ״�ȼ�ϵ��(DMFC)���ܳ�Ϊδ����Яʽ���Ӳ�ƷӦ�õ���������ͼ�Լ״�Ϊȼ�ϵ����͵����Ŀǰ�о��϶��һ�����������ȼ�ϵ�ع���ԭ��ʾ��ͼ���ش��������⣺

��1����ͼ�е�B���ϵĵ缫��ӦʽΪ______��
��2�����������״�ȼ�ϵ������Դ���ö��Ե缫���100mL��5mol��L-1����ͭ��Һ���������ռ���3.36L(��״��)����ʱ�����ļ״�������Ϊ____g����ʱ����������Һ�м���_____ʹ��Һ��ԭ����д���������ͭ��Һ�ĵ�ⷴӦ��ѧ����ʽ____��
��3�����������״�ȼ�ϵ������Դ����ʯī���缫��һ�������µ�ⱥ��ʳ��ˮ��ȡNaOH��

����ͼ���صĵ缫a�Ӽ״�ȼ�ϵ�ص�_____��( ��A��B) ��д�������ĵ缫��Ӧʽ��_____��������ʱ������һ�ֻ���ɫ���������(ClO2)��д����������ClO2�ĵ缫��Ӧʽ_________��
�ڵ��һ��ʱ�䣬�������������������Ϊ224 mL(��״��)ʱ��ֹͣ��⡣ͨ�������ӽ���Ĥ�������ӵ����ʵ���Ϊ_________mol��
���𰸡�CH3OH+O2-��6e-===CO2+4H+�� 3.2g CuO��CuCO3 2CuSO4+2H2O![]() 2H2SO4+2Cu+O2�� A 2Cl-��2e-�TCl2�� 2H2O+Cl-��5e-===ClO2+4H+ 0.02mol
2H2SO4+2Cu+O2�� A 2Cl-��2e-�TCl2�� 2H2O+Cl-��5e-===ClO2+4H+ 0.02mol
��������
��1������ͼʾ��B�Ǹ������״���B����ʧ�������ɶ�����̼�������ӣ�
��2���ö��Ե缫���100mL 5mol��L-1����ͭ��Һ������������������������ͭ���������ռ���3.36L(��״��)����ʱ��ת�Ƶ��ӵ����ʵ�����![]() 0.6mol�����ݵ�ʧ�����غ�������ļ״����������������ռ���0.15mol����ʱ�������õ�0.3molCu������Ԫ���غ������������Һ�м�������ʣ�
0.6mol�����ݵ�ʧ�����غ�������ļ״����������������ռ���0.15mol����ʱ�������õ�0.3molCu������Ԫ���غ������������Һ�м�������ʣ�
��3���ٸ����������ƶ������֪a��������������������ʧ��������������������������ʧ���ӿ��Բ���ClO2��
�ڵ���Ȼ�����Һʱ������������������������
��1������ͼʾ��B�Ǹ������״���B����ʧ�������ɶ�����̼�������ӣ�B�缫��Ӧʽ��CH3OH+O2-��6e-===CO2+4H+��
��2���ö��Ե缫���100mL 5mol��L-1����ͭ��Һ�����������������������ռ���3.36L(��״��)����ʱ��ת�Ƶ��ӵ����ʵ�����![]() 0.6mol�������ļ״�������Ϊxg�����ݵ�ʧ�����غ㣻
0.6mol�������ļ״�������Ϊxg�����ݵ�ʧ�����غ㣻
CH3OH+O2-��6e-===CO2+4H+
32g 6mol
Xg 0.6mol
X=3.2g
�������ռ���0.15mol����ʱ�������õ�0.3molCu������Ԫ���غ�,��������Һ�м���0.3molCuO��ʹ�������Һ��ԭ��
�������ͭ��Һ���ܷ�Ӧ��ѧ����ʽ��2CuSO4+2H2O![]() 2H2SO4+2Cu+O2����
2H2SO4+2Cu+O2����
��3�������������ƶ������֪a����������ԭ��ص�����������a�Ӽ״�ȼ�ϵ�ص�A����������������ʧ���������������缫��Ӧʽ��2Cl-��2e-�TCl2����������������ʧ���Ӳ���ClO2���缫��Ӧʽ��2H2O+Cl-��5e-===ClO2+4H+��
�ڵ������������������Ϊ224 mL(��״��)��0.01mol��ʱ��ת�Ƶ���0.02mol�����ݵ���غ㣬ͨ�������ӽ���Ĥ�������ӵ����ʵ���Ϊ0.02mol��

����Ŀ��PH3����ʳɱ�洦��ʱ���õ�Ѭ��ɱ�����ˮú���任ʱ������PH3��ʹ�����ж��������ѳ����ش��������⣺
��1��PH3ͨ��NaClO��Һ�ѳ�PH3ʱ������������һ�ֺ������ҷ�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ4��1����ú�����Ļ�ѧʽΪ______��
��2����֪���м������ݼ�P4�����ף����ӽṹ��
��ѧ�� | P-P | H-H | P-H |
|
����/��kJmol-1�� | 213 | 436 | 322 |
��Ӧ4PH3��g��P4��g��+6H2��g����H=______kJmol-1��ij�¶�ʱƽ����ϵ��c��PH3��=0.25molL-1��c��H2��=c��P4��=0.50molL-1����ƽ�ⳣ��K=______��
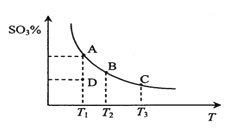
��3�����ױ�������Ӧ6.25CO2��g��+Fe3O4��s��+3PH3��g��=3FePO4��s��+4.5H2O��g��+6.25C��s����������ý�ж���������Ӧ����ƽ�ⳣ��Kp��KpΪ�Է�ѹ��ʾ��ƽ�ⳣ�����Ķ���ֵ���¶ȵĹ�ϵ��ͼ��ʾ��
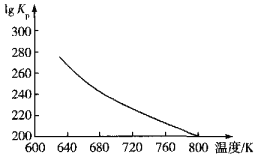
�ٸ÷�Ӧ����H______0���������������=������
��ͼ��lgKp=______[�г��÷�ѹp��CO2����p��PH3����p��H2O����ʾ�ļ���ʽ]��
��4����Ӧ��CH3��3AuPH3����CH3��AuPH3+C2H6���������£�
��һ������CH3��3AuPH3![]() ��CH3��3Au+PH3���췴Ӧ��
��CH3��3Au+PH3���췴Ӧ��
�ڶ�������CH3��3Au![]() C2H6+CH3Au������Ӧ��
C2H6+CH3Au������Ӧ��
��������CH3Au+PH3![]() ��CH3��AuPH3���췴Ӧ��
��CH3��AuPH3���췴Ӧ��
�ٷ�Ӧ���м������PH3��______��
�ڵ�______�����һ������������������Ӧ�Ļ�����
��5����Cu2+��Pd2+Һ���ѳ�PH3�ķ�ӦΪ��PH3+2O2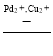 H3PO4������������ͬʱ�ܽ�����Һ��O2�����������PH3�ľ���Ч����ʱ��Ĺ�ϵ��ͼ��ʾ��
H3PO4������������ͬʱ�ܽ�����Һ��O2�����������PH3�ľ���Ч����ʱ��Ĺ�ϵ��ͼ��ʾ��
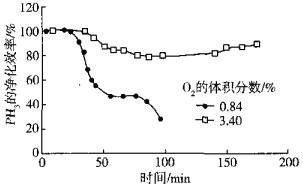
O2�����������PH3�ľ���Ч�ʸߵ�ԭ����______������ײ���۵�˵������