��Ŀ����
(15��)����ͼװ�ý���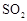 ת��Ϊ
ת��Ϊ ��ת���ʲⶨʵ�飺
��ת���ʲⶨʵ�飺
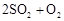

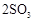 ��
��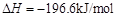 ����֪��
����֪��
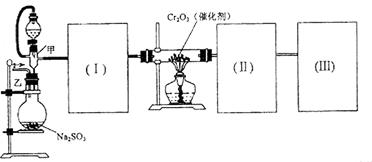
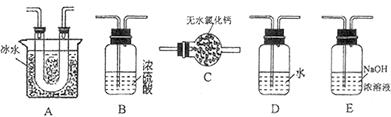
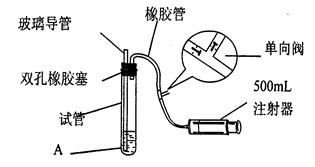
(1)Ҫ˳������ʵ�飬��ͼ��Ӧ���Ӻ��ʵ�װ�ã����ظ�ʹ�ã����������A��E��ѡ�����˵�װ�ã������������ո��ڡ�
(2)��װ�����Ӻã�ʵ��ǰ��������еIJ����ǣ�����д������̣� ��
(3)ʵ��ʱ��Ũ������˳��������ƿ�У���װ������������� ��
(4)��ʼʵ��ʱ���ȴ��Ҵ�����ͨ�� ��Ϊʹ
��Ϊʹ �нϸߵ�ת���ʣ�ʵ��ʱ����Ũ��������ȴ������Ⱥ�˳���� ��
�нϸߵ�ת���ʣ�ʵ��ʱ����Ũ��������ȴ������Ⱥ�˳���� ��
(5)ʵ���С��� �������ʱ��Ӧ���ƾ����ƿ�һ����ټ��ȡ����Է��¶ȹ��ߣ���������ԭ���� �����ô�������
�������ʱ��Ӧ���ƾ����ƿ�һ����ټ��ȡ����Է��¶ȹ��ߣ���������ԭ���� �����ô�������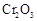 ���ķ�Ӧ��ʱ��
���ķ�Ӧ��ʱ��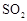 ��ת���ʻ� ������ߡ��������͡����䡱����
��ת���ʻ� ������ߡ��������͡����䡱����
(6)ʵ��ʱ����25.2g��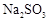 �������Ũ�����������ģ���Ӧ����ʱ����ͨ��
�������Ũ�����������ģ���Ӧ����ʱ����ͨ�� һ��ʱ�䣬�Ƶâ�װ�õ���������11.3g����ʵ����
һ��ʱ�䣬�Ƶâ�װ�õ���������11.3g����ʵ����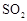 ��ת����Ϊ %������С�����һλ����
��ת����Ϊ %������С�����һλ����
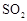 ת��Ϊ
ת��Ϊ ��ת���ʲⶨʵ�飺
��ת���ʲⶨʵ�飺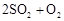

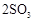 ��
��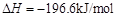 ����֪��
����֪��| | �۵㣨 �� �� | �е㣨 �� �� |
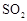 | -72.4 | -10 |
 | 16.8 | 44.3 |

(1)Ҫ˳������ʵ�飬��ͼ��Ӧ���Ӻ��ʵ�װ�ã����ظ�ʹ�ã����������A��E��ѡ�����˵�װ�ã������������ո��ڡ�

(2)��װ�����Ӻã�ʵ��ǰ��������еIJ����ǣ�����д������̣� ��
(3)ʵ��ʱ��Ũ������˳��������ƿ�У���װ������������� ��
(4)��ʼʵ��ʱ���ȴ��Ҵ�����ͨ��
 ��Ϊʹ
��Ϊʹ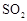 �нϸߵ�ת���ʣ�ʵ��ʱ����Ũ��������ȴ������Ⱥ�˳���� ��
�нϸߵ�ת���ʣ�ʵ��ʱ����Ũ��������ȴ������Ⱥ�˳���� ��(5)ʵ���С���
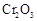 �������ʱ��Ӧ���ƾ����ƿ�һ����ټ��ȡ����Է��¶ȹ��ߣ���������ԭ���� �����ô�������
�������ʱ��Ӧ���ƾ����ƿ�һ����ټ��ȡ����Է��¶ȹ��ߣ���������ԭ���� �����ô�������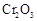 ���ķ�Ӧ��ʱ��
���ķ�Ӧ��ʱ��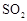 ��ת���ʻ� ������ߡ��������͡����䡱����
��ת���ʻ� ������ߡ��������͡����䡱����(6)ʵ��ʱ����25.2g��
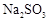 �������Ũ�����������ģ���Ӧ����ʱ����ͨ��
�������Ũ�����������ģ���Ӧ����ʱ����ͨ�� һ��ʱ�䣬�Ƶâ�װ�õ���������11.3g����ʵ����
һ��ʱ�䣬�Ƶâ�װ�õ���������11.3g����ʵ����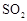 ��ת����Ϊ %������С�����һλ����
��ת����Ϊ %������С�����һλ����(1)`��B ��A ��BE(��1�֣���3��)
(2)���װ�õ�������(2��)
(3)���ַ�Һ©����ѹǿ����ƿ��ѹǿ���(2��)
(4)�ȼ��ȴ�����Ȼ�����μ�Ũ����(2��)
(5)�¶ȹ��߲�����SO3�����ɣ���Ӱ������Ļ���(2��) ����(2��)
(6)70.6��(2��)
(2)���װ�õ�������(2��)
(3)���ַ�Һ©����ѹǿ����ƿ��ѹǿ���(2��)
(4)�ȼ��ȴ�����Ȼ�����μ�Ũ����(2��)
(5)�¶ȹ��߲�����SO3�����ɣ���Ӱ������Ļ���(2��) ����(2��)
(6)70.6��(2��)
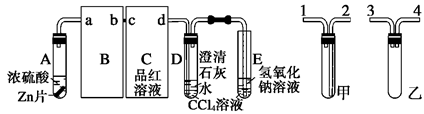
�ۺ������Ϣ��ʵ��װ���������ҷֱ�Ϊ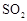 �ķ���װ������
�ķ���װ������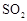 ����Ũ������I��������������ɵ�SO3�ľ���ˮ������II����U���У�Ϊ�˱���SO3��ˮ��SO2��Ⱦ�������ڣ�III�������Ũ�������ˮװ�ü�NaOH��β������װ�á�
����Ũ������I��������������ɵ�SO3�ľ���ˮ������II����U���У�Ϊ�˱���SO3��ˮ��SO2��Ⱦ�������ڣ�III�������Ũ�������ˮװ�ü�NaOH��β������װ�á�
��4��Ϊ��ֹSO2����ʧӦ����Ԥ�ȴ�����Ȼ�����μ�Ũ����
��5��2SO2��O2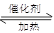 2SO3������Ӧ�Ƿ��ȷ�Ӧ���¶ȹ��߲�����SO3�����ɣ���Ӱ������Ļ��ԡ�
2SO3������Ӧ�Ƿ��ȷ�Ӧ���¶ȹ��߲�����SO3�����ɣ���Ӱ������Ļ��ԡ�
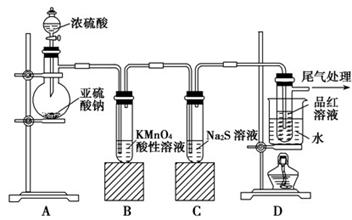
��6��Na2SO3 �� SO2�� SO3
�ɣ�25.2g 16g
�ʣ�SO2��ת����Ϊ��11.3/16 = 70.6��
 �ķ���װ������
�ķ���װ������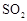 ����Ũ������I��������������ɵ�SO3�ľ���ˮ������II����U���У�Ϊ�˱���SO3��ˮ��SO2��Ⱦ�������ڣ�III�������Ũ�������ˮװ�ü�NaOH��β������װ�á�
����Ũ������I��������������ɵ�SO3�ľ���ˮ������II����U���У�Ϊ�˱���SO3��ˮ��SO2��Ⱦ�������ڣ�III�������Ũ�������ˮװ�ü�NaOH��β������װ�á���4��Ϊ��ֹSO2����ʧӦ����Ԥ�ȴ�����Ȼ�����μ�Ũ����
��5��2SO2��O2
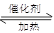 2SO3������Ӧ�Ƿ��ȷ�Ӧ���¶ȹ��߲�����SO3�����ɣ���Ӱ������Ļ��ԡ�
2SO3������Ӧ�Ƿ��ȷ�Ӧ���¶ȹ��߲�����SO3�����ɣ���Ӱ������Ļ��ԡ���6��Na2SO3 �� SO2�� SO3
�ɣ�25.2g 16g
�ʣ�SO2��ת����Ϊ��11.3/16 = 70.6��

��ϰ��ϵ�д�
�����Ŀ
 ��
��