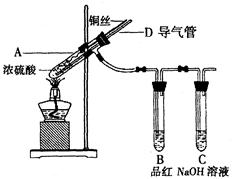
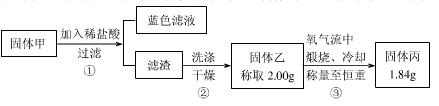
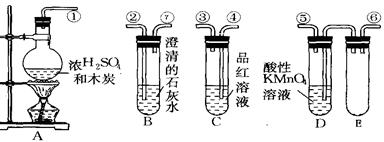
��Ŀ����
��8�֣�����������������Ԫ�أ����������������������������Ҫ���á�
��1�������е����Ҫ��Դ֮һ�Ǽӵ�ʳ�Ρ��ӵ�ʳ���еĵ�Ԫ���Ե���أ�KIO3������ʽ���ڣ����Ԫ�صĻ��ϼ���__________���ӵ�ʳ�ε�ʳ�÷����ǡ���ʳƷ�����롱��˵�������������ʱ��__________��
��2��������̦�к��зḻ�ĵ�Ԫ�أ�Ҳ��������һ����Դ��ȡ�����ҵĽ�ȡҺ�������ữ���ټ�������H2O2��Һ�������Һ����Һ������֤�������к��е�Ԫ�ء���Ӧ�����ӷ���ʽ��________��
��3��2012��3�£����������¹涨ʳ���е⺬����Ϊ��18~33��mg��kg��Ϊ�ⶨ�ӵ�ʳ����Ʒ�ĵ⺬����ijʵ��С����������²��裺
I. ����ƽȷ��ȡ10.0 gʳ����Ʒ������������ˮʹ����ȫ�ܽ⡣
II. ��ϡ�����ữ������Һ����������KI��Һ��ʹKIO3��KI��Ӧ��ȫ��
III. �Ե���Ϊָʾ������μ������ʵ���Ũ��Ϊ2.0��10��3mol��L��1��Na2S2O3��Һ6.0 mL��ǡ�÷�Ӧ��ȫ������֪��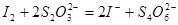 ��
��
�ٲ���II�з�Ӧ�����ӷ���ʽ��__________��
�ڲ���III���жϷ�Ӧǡ����ȫ��������__________��
������ʵ����Ʒ�е⺬����__________mg��kg�����ϱ����й涨��
��1�������е����Ҫ��Դ֮һ�Ǽӵ�ʳ�Ρ��ӵ�ʳ���еĵ�Ԫ���Ե���أ�KIO3������ʽ���ڣ����Ԫ�صĻ��ϼ���__________���ӵ�ʳ�ε�ʳ�÷����ǡ���ʳƷ�����롱��˵�������������ʱ��__________��
��2��������̦�к��зḻ�ĵ�Ԫ�أ�Ҳ��������һ����Դ��ȡ�����ҵĽ�ȡҺ�������ữ���ټ�������H2O2��Һ�������Һ����Һ������֤�������к��е�Ԫ�ء���Ӧ�����ӷ���ʽ��________��
��3��2012��3�£����������¹涨ʳ���е⺬����Ϊ��18~33��mg��kg��Ϊ�ⶨ�ӵ�ʳ����Ʒ�ĵ⺬����ijʵ��С����������²��裺
I. ����ƽȷ��ȡ10.0 gʳ����Ʒ������������ˮʹ����ȫ�ܽ⡣
II. ��ϡ�����ữ������Һ����������KI��Һ��ʹKIO3��KI��Ӧ��ȫ��
III. �Ե���Ϊָʾ������μ������ʵ���Ũ��Ϊ2.0��10��3mol��L��1��Na2S2O3��Һ6.0 mL��ǡ�÷�Ӧ��ȫ������֪��
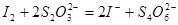 ��
���ٲ���II�з�Ӧ�����ӷ���ʽ��__________��
�ڲ���III���жϷ�Ӧǡ����ȫ��������__________��
������ʵ����Ʒ�е⺬����__________mg��kg�����ϱ����й涨��
��1����5��1�֣��ֽ⣨1�֣�
��2��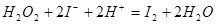 ��2�֣�����ƽ��1�֣�
��2�֣�����ƽ��1�֣�
��3����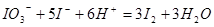 ��2�֣�����ƽ��1�֣�
��2�֣�����ƽ��1�֣�
����ɫ��ȥ��1�֣� ��25.4��1�֣�25���۷֣�
��2��
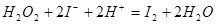 ��2�֣�����ƽ��1�֣�
��2�֣�����ƽ��1�֣���3����
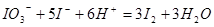 ��2�֣�����ƽ��1�֣�
��2�֣�����ƽ��1�֣�����ɫ��ȥ��1�֣� ��25.4��1�֣�25���۷֣�
��1���������K��O�Ļ��ϼ۷ֱ��ǣ�1�ۺͣ�2�ۣ����Ե�Ļ��ϼ���2��3-1=+5�ۡ�����������ֽ⣬����Ӧ���ǡ���ʳƷ�����롱��
��2��˫��ˮ���������ԣ������������ӣ����ɵ��ʵ⣬����ʽΪ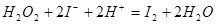 ��
��
��3������������Һ�У�������������⻯�����ɵ��ʵ⣬����ʽΪ
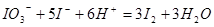 ��
��
�����ڵ�͵��۷�����ɫ��Ӧ����������ɫ�������յ�ʱ������ʱ��ɫ��ȥ��
�۸��ݷ���ʽ��֪KIO3��3I2��6Na2S2O3�����Ե���ص����ʵ�����2.0��10��3mol��L��1��0.006L��6��2.0��10��6mol��������Ʒ�е⺬����2.0��10��6��127��1000mg��0.01kg��25.4mg��kg��
��2��˫��ˮ���������ԣ������������ӣ����ɵ��ʵ⣬����ʽΪ
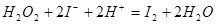 ��
����3������������Һ�У�������������⻯�����ɵ��ʵ⣬����ʽΪ
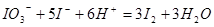 ��
�������ڵ�͵��۷�����ɫ��Ӧ����������ɫ�������յ�ʱ������ʱ��ɫ��ȥ��
�۸��ݷ���ʽ��֪KIO3��3I2��6Na2S2O3�����Ե���ص����ʵ�����2.0��10��3mol��L��1��0.006L��6��2.0��10��6mol��������Ʒ�е⺬����2.0��10��6��127��1000mg��0.01kg��25.4mg��kg��

��ϰ��ϵ�д�
�����Ŀ
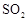 ת��Ϊ
ת��Ϊ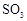 ��ת���ʲⶨʵ�飺
��ת���ʲⶨʵ�飺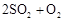
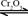
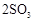 ��
��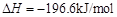 ����֪��
����֪��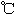 ��
��
 ��Ϊʹ
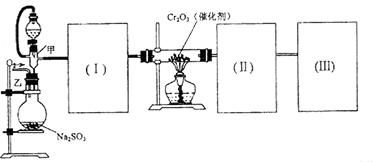
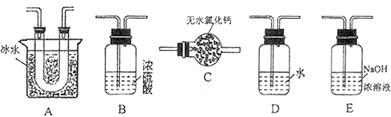
��Ϊʹ �������ʱ��Ӧ���ƾ����ƿ�һ����ټ��ȡ����Է��¶ȹ��ߣ���������ԭ���� �����ô�������
�������ʱ��Ӧ���ƾ����ƿ�һ����ټ��ȡ����Է��¶ȹ��ߣ���������ԭ���� �����ô�������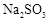 �������Ũ�����������ģ���Ӧ����ʱ����ͨ��
�������Ũ�����������ģ���Ӧ����ʱ����ͨ��