��Ŀ����
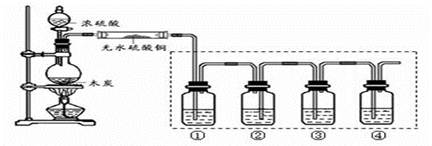
�������֣�ijͬѧ������ͼ1��ʾ��װ����̽��SO2�����ʼ��й�ʵ�顣
��1��ʵ��ǰӦ�ȼ���װ�õ������ԣ�������
��2��ʵ�������������ƹ��������ᷴӦ��ȡSO2���壬д���÷�Ӧ�Ļ�ѧ����ʽ
��
��3���ֱ�SO2����ͨ����������C��Һ�У���ش��������⣺
������SO2ͨ����ɫʯ����Һ�������� ������ͨ�������SO2���壬������ ��
��SO2ͨ���Ϻ�ɫKMnO4��Һ�������� ��
��SO2����ͨ�����ʯ��ˮ�У����� ��
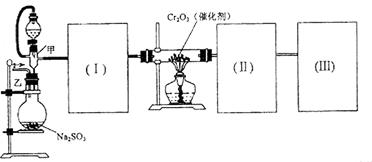
��4����ͬѧ��ȡ��SO2�����л���CO2���壬�������ʵ��֤����������м���SO2���壬����CO2���塣����ѡ����ͼ2ϴ��װ�����ʵ�顣ϴ��װ�������Һ�ǣ�
AŨ���ᡢB����������Һ��C����ͭ��Һ��DƷ����Һ��
E����ʯ��ˮ��F���������Һ��G̼��������Һ��
����װ�õ���װ�Լ�����˳���ǣ�����ĸ��ʾ������װ�ÿ����ظ�ʹ�ã���Щװ��Ҳ���Բ��ã� ��
��1��ʵ��ǰӦ�ȼ���װ�õ������ԣ�������
��2��ʵ�������������ƹ��������ᷴӦ��ȡSO2���壬д���÷�Ӧ�Ļ�ѧ����ʽ
��
��3���ֱ�SO2����ͨ����������C��Һ�У���ش��������⣺
������SO2ͨ����ɫʯ����Һ�������� ������ͨ�������SO2���壬������ ��
��SO2ͨ���Ϻ�ɫKMnO4��Һ�������� ��
��SO2����ͨ�����ʯ��ˮ�У����� ��
��4����ͬѧ��ȡ��SO2�����л���CO2���壬�������ʵ��֤����������м���SO2���壬����CO2���塣����ѡ����ͼ2ϴ��װ�����ʵ�顣ϴ��װ�������Һ�ǣ�
AŨ���ᡢB����������Һ��C����ͭ��Һ��DƷ����Һ��
E����ʯ��ˮ��F���������Һ��G̼��������Һ��
����װ�õ���װ�Լ�����˳���ǣ�����ĸ��ʾ������װ�ÿ����ظ�ʹ�ã���Щװ��Ҳ���Բ��ã� ��

��1���رշ�Һ©���Ļ�������˫�֣�����ë������סB��C �������ݲ������ƿ�˫�֣�C�е����γ�һ��ˮ����˵����©���������֣�
��2��Na2SO3+H2SO4��Na2SO4+SO2��+H2O�����֣�
��3������ɫ��Һ��Ϊ��ɫ ��ɫ����ȥ������Һ��ɫ�ޱ仯�������֣�
���Ϻ�ɫ��ȥ ��3�֣�
�������а�ɫ�������ɣ�Ȼ���ɫ������ʧ�����֣�
��4��D��F��D��E��D��F��F��E������˳�����Ҳ���֣������֣�
��2��Na2SO3+H2SO4��Na2SO4+SO2��+H2O�����֣�
��3������ɫ��Һ��Ϊ��ɫ ��ɫ����ȥ������Һ��ɫ�ޱ仯�������֣�
���Ϻ�ɫ��ȥ ��3�֣�
�������а�ɫ�������ɣ�Ȼ���ɫ������ʧ�����֣�
��4��D��F��D��E��D��F��F��E������˳�����Ҳ���֣������֣�
��1�����������Եķ����ǣ����ȹرշ�Һ©���Ļ�����Ȼ����˫�֣�����ë������סB��C�������ݲ������ƿ�˫�֣�C�е����γ�һ��ˮ����˵����©����
��2�����������ǿ��������ģ����������������Ʒ�Ӧ�����������ơ�SO2��ˮ����ӦʽΪNa2SO3+H2SO4��Na2SO4+SO2��+H2O��
��3����SO2����ˮ���������ᣬ��Һ�����ԣ�������Һ�Ժ�ɫ������SO2��Ư�ײ���ʹ���ָʾ����ɫ�����Լ���ͨ��SO2����Һ����ɫҲ����仯��
��SO2���л�ԭ�ԣ����������Һ���������ԣ����߷���������ԭ��Ӧ��������Һ���Ϻ�ɫ����ȥ��
��SO2��������������������Ʒ�Ӧ����������ư�ɫ������ˮ������ͨ��SO2����ɫ��������ʧ���ܽ⡣
��4������SO2һ����Ʒ����Һ������CO2һ���ó����ʯ��ˮ��������SO2Ҳ���dz����ʯ��ˮ����ǣ������Լ���SO2������ͨ������ʯ��ˮ֮ǰ����Ӧ��ȥSO2��Ϊ�˷�ֹSO2������������Ҫ�ٴ���Ʒ����Һ��������SO2�Ƿ���ȫ��������������ȷ�Ĵ���D��F��D��E��D��F��F��E��
��2�����������ǿ��������ģ����������������Ʒ�Ӧ�����������ơ�SO2��ˮ����ӦʽΪNa2SO3+H2SO4��Na2SO4+SO2��+H2O��
��3����SO2����ˮ���������ᣬ��Һ�����ԣ�������Һ�Ժ�ɫ������SO2��Ư�ײ���ʹ���ָʾ����ɫ�����Լ���ͨ��SO2����Һ����ɫҲ����仯��
��SO2���л�ԭ�ԣ����������Һ���������ԣ����߷���������ԭ��Ӧ��������Һ���Ϻ�ɫ����ȥ��
��SO2��������������������Ʒ�Ӧ����������ư�ɫ������ˮ������ͨ��SO2����ɫ��������ʧ���ܽ⡣
��4������SO2һ����Ʒ����Һ������CO2һ���ó����ʯ��ˮ��������SO2Ҳ���dz����ʯ��ˮ����ǣ������Լ���SO2������ͨ������ʯ��ˮ֮ǰ����Ӧ��ȥSO2��Ϊ�˷�ֹSO2������������Ҫ�ٴ���Ʒ����Һ��������SO2�Ƿ���ȫ��������������ȷ�Ĵ���D��F��D��E��D��F��F��E��

��ϰ��ϵ�д�
�����Ŀ
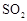 ת��Ϊ
ת��Ϊ ��ת���ʲⶨʵ�飺
��ת���ʲⶨʵ�飺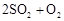

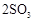 ��
��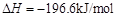 ����֪��
����֪�� ��
��
 ��Ϊʹ
��Ϊʹ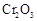 �������ʱ��Ӧ���ƾ����ƿ�һ����ټ��ȡ����Է��¶ȹ��ߣ���������ԭ���� �����ô�������
�������ʱ��Ӧ���ƾ����ƿ�һ����ټ��ȡ����Է��¶ȹ��ߣ���������ԭ���� �����ô�������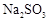 �������Ũ�����������ģ���Ӧ����ʱ����ͨ��
�������Ũ�����������ģ���Ӧ����ʱ����ͨ��