��Ŀ����
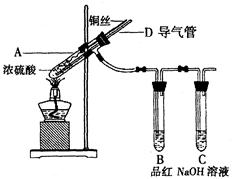
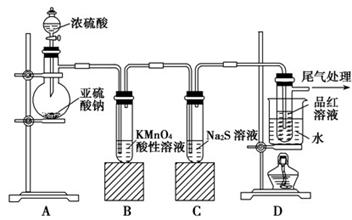
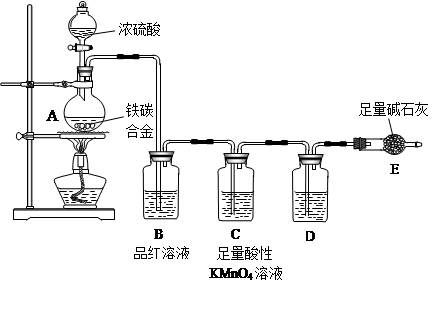
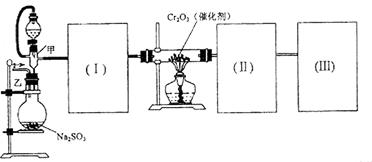
��14�֣�ij��ѧ��ȤС��Ϊ̽��SO2�����ʣ�����ͼ��ʾװ�ý���ʵ�顣
��ش��������⣺
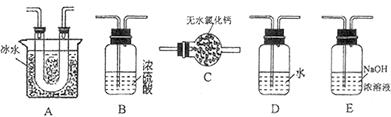
(1)װ��A��ʢ���������Ƶ�����������________��
(2)ʵ������У�װ��B�в�����������________________________���������˵��SO2���е�������________________��
(3)װ��C��Na2S��Һ�ڿ����в��ױ��棬ʱ�䳤�˻����ǣ�ԭ����(�����ӷ���ʽ��ʾ) __________________________________��
(4)װ��D��Ŀ����̽��SO2��Ʒ�����õĿ����ԣ���д��ʵ�����������__________________________________��
(5)����ѷ�Һ©���е��е�ŨH2SO4����ŨHNO3���Դ�ʵ���Ƿ���Ӱ��
________����ǡ��� ������˵���������_____________________________
(6)��ҵ���û�ͭ�� CuFeS2��ұ��ͭ������Ʒ��Ҳ��SO2 ��ұ��ͭ�ķ�ӦΪ
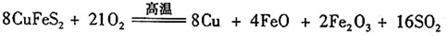
��CuFeS2�� Fe �Ļ��ϼ�Ϊ��2 ����Ӧ�б���ԭ��Ԫ���� ����Ԫ�ط��ţ���������0.8 molͭʱ���˷�Ӧת�Ƶĵ�����Ŀ��___________________��

��ش��������⣺
(1)װ��A��ʢ���������Ƶ�����������________��
(2)ʵ������У�װ��B�в�����������________________________���������˵��SO2���е�������________________��
(3)װ��C��Na2S��Һ�ڿ����в��ױ��棬ʱ�䳤�˻����ǣ�ԭ����(�����ӷ���ʽ��ʾ) __________________________________��
(4)װ��D��Ŀ����̽��SO2��Ʒ�����õĿ����ԣ���д��ʵ�����������__________________________________��
(5)����ѷ�Һ©���е��е�ŨH2SO4����ŨHNO3���Դ�ʵ���Ƿ���Ӱ��
________����ǡ��� ������˵���������_____________________________
(6)��ҵ���û�ͭ�� CuFeS2��ұ��ͭ������Ʒ��Ҳ��SO2 ��ұ��ͭ�ķ�ӦΪ
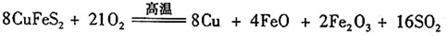
��CuFeS2�� Fe �Ļ��ϼ�Ϊ��2 ����Ӧ�б���ԭ��Ԫ���� ����Ԫ�ط��ţ���������0.8 molͭʱ���˷�Ӧת�Ƶĵ�����Ŀ��___________________��
(1)������ƿ��1�֣� (2) ��Һ��ɫ����ɫ����ȥ����ԭ�ԣ�ÿ��1�֣���2�֣���
(3) 2S2-��O2��2 H2O ===2 S����4 OH����2�֣�
(4)Ʒ����Һ��ɫ�رշ�Һ©������������ȼ�ƾ��Ƽ��ȣ���Һ�ָ�Ϊ��ɫ��2�֣�
(5)�ǣ�1�֣�ŨHNO3�ɽ�������������Ϊ�����ƣ����ò���SO2���壨2�֣�
��6�� Cu ��O��2�֣���ֻдһ�����÷֣� 10NA��6.02��1024��2�֣�
(3) 2S2-��O2��2 H2O ===2 S����4 OH����2�֣�
(4)Ʒ����Һ��ɫ�رշ�Һ©������������ȼ�ƾ��Ƽ��ȣ���Һ�ָ�Ϊ��ɫ��2�֣�
(5)�ǣ�1�֣�ŨHNO3�ɽ�������������Ϊ�����ƣ����ò���SO2���壨2�֣�
��6�� Cu ��O��2�֣���ֻдһ�����÷֣� 10NA��6.02��1024��2�֣�
��1�����������Ĺ�����жϣ���������������ƿ��
��2��A�в���SO2������SO2���л�ԭ�ԣ������Ը��������Һ���������ԣ�����B����Һ��ɫ����ɫ����ȥ��
��3����������Ԫ�صĻ��ϼ�����ͼۣ�2�ۣ����л�ԭ�ԣ����ױ����������ɵ�������ʽΪ2S2-��O2��2 H2O ===2 S����4 OH����
��4��SO2��Ư����ԭ���Ǻ���ɫ���ʽ�ϣ����ɲ��ȶ�����ɫ���ʡ��ڼ��ȵ������£����ָܻ�ԭ������ɫ���ݴ˿��Լ��顣��Ʒ����Һ��ɫ�رշ�Һ©������������ȼ�ƾ��Ƽ��ȣ���Һ�ָ�Ϊ��ɫ��
��5������Ũ�������ǿ�����ԣ�ŨHNO3�ɽ�������������Ϊ�����ƣ����ò���SO2���壬��˶�ʵ�����Ӱ�졣
��6��CuFeS2�� Fe �Ļ��ϼ�Ϊ��2 ����Cu�Ļ��ϼ��ǣ�2�ۣ�S�Ļ��ϼ��ǣ�2�ۡ�����������Cu�Ļ��ϼ���0�ۣ��õ����ӣ�����ԭ��ͬʱ����Ҳ������������Ԫ�ر���ԭ��������0.8 molͭʱ������ԭ��ͭ��0.8mol��������2.1mol������ת�Ƶ�����0.8mol��2��2.1mol��4��10mol����ת�Ƶ��ӵĸ�����10NA��6.02��1024��
��2��A�в���SO2������SO2���л�ԭ�ԣ������Ը��������Һ���������ԣ�����B����Һ��ɫ����ɫ����ȥ��
��3����������Ԫ�صĻ��ϼ�����ͼۣ�2�ۣ����л�ԭ�ԣ����ױ����������ɵ�������ʽΪ2S2-��O2��2 H2O ===2 S����4 OH����
��4��SO2��Ư����ԭ���Ǻ���ɫ���ʽ�ϣ����ɲ��ȶ�����ɫ���ʡ��ڼ��ȵ������£����ָܻ�ԭ������ɫ���ݴ˿��Լ��顣��Ʒ����Һ��ɫ�رշ�Һ©������������ȼ�ƾ��Ƽ��ȣ���Һ�ָ�Ϊ��ɫ��
��5������Ũ�������ǿ�����ԣ�ŨHNO3�ɽ�������������Ϊ�����ƣ����ò���SO2���壬��˶�ʵ�����Ӱ�졣
��6��CuFeS2�� Fe �Ļ��ϼ�Ϊ��2 ����Cu�Ļ��ϼ��ǣ�2�ۣ�S�Ļ��ϼ��ǣ�2�ۡ�����������Cu�Ļ��ϼ���0�ۣ��õ����ӣ�����ԭ��ͬʱ����Ҳ������������Ԫ�ر���ԭ��������0.8 molͭʱ������ԭ��ͭ��0.8mol��������2.1mol������ת�Ƶ�����0.8mol��2��2.1mol��4��10mol����ת�Ƶ��ӵĸ�����10NA��6.02��1024��

��ϰ��ϵ�д�
 ���ĺ����Ͼ�������ϵ�д�
���ĺ����Ͼ�������ϵ�д�
�����Ŀ
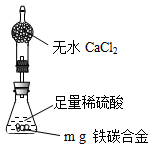
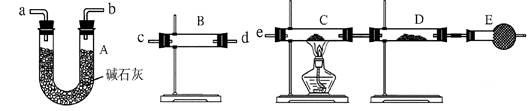
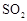 ת��Ϊ
ת��Ϊ ��ת���ʲⶨʵ�飺
��ת���ʲⶨʵ�飺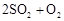

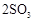 ��
��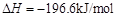 ����֪��
����֪�� ��
��
 ��Ϊʹ
��Ϊʹ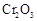 �������ʱ��Ӧ���ƾ����ƿ�һ����ټ��ȡ����Է��¶ȹ��ߣ���������ԭ���� �����ô�������
�������ʱ��Ӧ���ƾ����ƿ�һ����ټ��ȡ����Է��¶ȹ��ߣ���������ԭ���� �����ô�������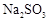 �������Ũ�����������ģ���Ӧ����ʱ����ͨ��
�������Ũ�����������ģ���Ӧ����ʱ����ͨ��