��Ŀ����
Ϊ��֤±�ص��������Ե����ǿ��,ijС������ͼ��ʾװ�ý���ʵ��(�г���������ȥ,�������Ѽ���)��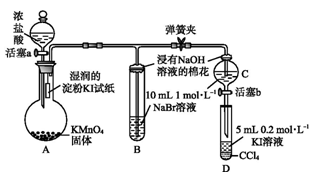
ʵ�����:
��.���ɼ�,����a,�μ�Ũ���ᡣ
��.��B��C�е���Һ����Ϊ��ɫʱ,�н����ɼС�
��.��B����Һ�ɻ�ɫ��Ϊ�غ�ɫʱ,�رջ���a��
��.����
(1)A�в�������ɫ����,�仯ѧ����ʽ������������������������������
(2)��֤������������ǿ�ڵ��ʵ������������������������������
(3)B����Һ������Ӧ�����ӷ���ʽ����������������
(4)Ϊ��֤���������ǿ�ڵ�,���̢��IJ�������������������������������������
(5)���̢�ʵ���Ŀ����������������������������������������
(6)�ȡ��塢�ⵥ�ʵ�������������ԭ��:ͬ����Ԫ�ش��ϵ�����������,�õ�������������
(1)16HCl(Ũ)+2KMnO4=2MnCl2+2KCl+5Cl2��+8H2O
(2)����KI��ֽ����
(3)Cl2+2Br-=Br2+2Cl-
(4)����b,������C����Һ����D��,�رջ���b,ȡ��D�����ú�CCl4����Һ��Ϊ�Ϻ�ɫ
(5)ȷ��C�Ļ�ɫ��Һ����Cl2,�ų�Cl2�����û���ʵ��ĸ���
(6)ԭ�Ӱ뾶������
����

��14�֣�2013��6�£��ҹ������������ٴ�ˢ�¡��й���ȡ�������DZ7062�ף�Ϊ�ҹ��������Դ�Ŀ����춨�˻�����������зḻ���̽�˿��̽�˵���Ҫ�ɷ���MnO2��ͬʱ�����л�ͭ��
�����������������������ѺϽ�����Ƴɣ���������7000m����г�����ѹ��Ti�����Ѱۣ�TiO2��Ϊԭ�Ͻ����������Ѱ�������TiO2������ˮ���������ᣨH2TiO3�������������ճ����Ƶõġ�TiO2������ˮ������ӷ���ʽΪ____________________________��
��MnO2��һ����Ҫ�������ܲ��ϣ���ҵ�ϴ��̽������ȡ������MnO2������������ͼ��ʾ��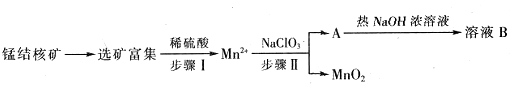
��1�����������NaClO3Ϊ��������������0.05molMnO2ʱ������0.1mol/L��NaClO3��Һ200ml���÷�Ӧ���ӷ���ʽΪ_______________________________��
��2����֪��ҺB������֮һ��ѭ���������������������ʵ�������____________��
�����û�ͭ����ͭ������¯������Fe2O3��FeO��SiO2��Al2O3�ȣ����Ʊ�Fe2O3������Ϊ��
��A���ù�����ϡ�����ȡ¯�������ˣ���B������Һ�м���5����H2O2���������м��������NaOH��Һ�����ˣ�������ϴ�ӡ�������յõ�Fe2O3������������Ϣ�ش��������⣺
��1����B��������Һ�м���5%��H2O2����Ŀ����_________________________________��
��2�����ʵ��֤��¯���к���FeO___________________________________________��
��3�������յõ���Fe2O3��ԭΪFe���ʣ��ٽ�����Ϊm g��Fe���ʷֳ���ȵ��ķݣ��ֱ���50mL��100mL��150mL��200mL�ĵ�Ũ�ȵ�ϡ���ᷴӦ����Ӧ����NO�ڱ���µ������������
| ʵ�� | �� | �� | �� | �� |
| V(HNO3)/ml | 50 | 100 | 150 | 200 |
| V(NO)/L | 1.344 | 2.688 | 3.36 | 3.36 |
��д��ʵ��ڷ�����Ӧ�Ļ�ѧ����ʽ��_____________________________��
ij��ѧС����ѧϰԪ��������֪ʶ�Խ̲���Cl2��Fe2��������Fe3����ʵ���һ��˼������������⣺Cl2�ܽ�Fe2��������Fe3������ôBr2��I2�ܷ�Fe2��������Fe3����
����һ�������Ʋ⡣
����ͬѧ��ΪBr2��I2���ܽ�Fe2��������Fe3����������____________________
_________________________________________________________________��
����ͬѧ��ΪBr2��I2�����ܽ�Fe2��������Fe3��������ͬѧ��ΪBr2�ܽ�
Fe2��������Fe3������I2���ܣ�������ͬһ������ϵ���±�ص��ʵ�������������
���ڶ������ʵ�������֤��
����Թ��м����������ۣ�����10 mLϡ���ᣬ���Թܣ���ַ�Ӧ��������ʣ�࣬ȡ�ϲ���Һ��������ʵ�顣
ʵ��1��
| �Թ� | ���� | ���� |
| �� | �����Թ��м���2 mL���Ƶ�FeCl2��Һ�������Թ��еμ���������ɫ����ˮ�����Թ� | ��ҺΪ ��ɫ |
| �� | �����Թ��м���2 mL���Ƶ�FeCl2��Һ�������Թ��еμ������ػ�ɫ�ĵ�ˮ�����Թ� | ��ҺΪ ��ɫ |
��������ʵ������ķ�������͡�
��1����ͬѧ��Ϊ�Թܢ��е�����˵����ˮ�ܽ�Fe2����������Ӧ�����ӷ���ʽΪ_________________________________________________________________��
��ͬѧ��ΪӦ�ò���ʵ�飬���ܵó���ͬѧ�Ľ��ۡ����������ͬѧ���ʵ�飺
ʵ��2��
| ���� | ���� |
| | |
��2����С��ͬѧ���Թܢ������õ���Һ�ʻ�ɫ��ԭ��չ�����ۣ�����������ּ��裺
����1����ˮ��FeCl2��Һ����Ӧ����ɫ�ǵ�ˮϡ�ͺ����ɫ��
����2.________________��
ʵ��3������ʵ�����жϼ����Ƿ������
| ���� | ���� |
| ���Թܢ����õ���Һ�м�������0.5 mL CCl4�����������һ��ʱ�䡣ȡ���ϲ���Һ���μ�KSCN��Һ | ���ú��ϲ���Һ������ɫ���²���ҺΪ��ɫ��ȡ�ϲ���Һ�μ�KSCN��Һ����Һ��dz��ɫ |
��ͬѧ��Ϊʵ��3���������˵������2��������ͬѧ��Ϊ���Ͻ������������ʵ��4����̽����
ʵ��4��
| ���� | ���� |
| ����һ֧�Թ��м���2 mL���Ƶ�FeCl2��Һ���μ�0.5 mL��ˮ���ټ���0.5 mL�������������������һ��ʱ�䡣ȡ���²���Һ���μ�KSCN��Һ | ���ú��ϲ���ҺΪ��ɫ���²���Һ������ɫ��ȡ�²���Һ�������еμ�KSCN��Һ����Һû�г�dz��ɫ |
����Ϊʵ��4�м���������������ҪĿ����___________________________��
��ͬѧ����ʵ��4������ó����ۣ��ڱ���ʵ�������£���ˮ��FeCl2��Һ��Ӧ�ij̶Ⱥ�С��
��3��Cl2��Br2��I2����Fe2����������������ԭ�ӽṹ�ǶȽ���ԭ��______________________________________________________________________��
��������������Ԫ��֮һ���С�����Ԫ�ء�֮�ơ�ʳ�üӵ�ʳ�ο�Ԥ����ȱ��������������֪���������������£�I���ܱ�NO3-����������IO3-���ӣ���H2O2��O2��������I2����IO3-�����ܱ�HSO3-���ӻ�ԭ��I2��
��������ʵ���ҳ���������������ѡ�Լ��������о�ijʳ����Ʒ�����ӵ�Ĵ�����ʽ��I2��I-��IO3-�е���һ�֡�
��ѡ�Լ����£�1.0 mol?L-1HNO3��Һ��1.0 mol?L-1 H2SO4��Һ��1.0 mol?L-1NaHSO3��Һ��3% H2O2��Һ��1%������Һ������ˮ
���������
����1����ʳ����Ʒ�к�I2 ��
����2����ʳ����Ʒ�к�I��
����3�� ��
����Ʒ�����ʵ��̽��
������ʳ����Ʒ��������ˮ�Ƴ���Һ���밴Ҫ����д�±�
| ʵ�鲽�� | Ԥ����������� |
| ����1��ȡ����������Һע���Թ��У����뼸�ε�����Һ�� | ����Һ�� �������1������������1���������ٽ��в���2 |
| ����2�� | ����Һ����ɫ�������2��������Ӧ�����ӷ���ʽΪ ��������2���������ٽ��в���3 |
| ����3�� | |
��������˼��
����KIO3��KI��������Ϊʳ�üӵ����е����Դ���ӻ�ѧ�Ƕ����������������� ���KIO3����KI�������ã������� ��
þ������Ϸ�ĩ10.2g����������500mL4mol/L���������Ҫʹ���������ﵽ���ֵ���������2mol��l��1������������Һ�����Ϊ
| A��1000mL | B��500mL | C��100mL | D��1500mL |