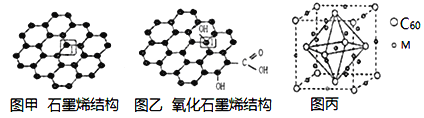
��Ŀ����
����Ŀ��ijѧ����0.2000mol��L��1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ������ɷ�Ϊ���¼�����
�� ������ˮϴ�Ӽ�ʽ�ζ��ܺ�����ע��NaOH��Һ����0���̶������ϣ�
�� �̶��õζ��ܲ�ʹ�ζ��ܼ������Һ�壻
�� ����Һ������0������0���̶������£������¶�����
�� ��ȡ20.00mL����Һע��ྻ����ƿ�У�������3�η�̪��Һ��
�� �ñ�Һ�ζ����յ㣬���µζ���Һ�������
��ش�
��1�����ϲ�����������һ���д�����ָ�����___���ô�������ᵼ�²ⶨ���___(����ƫ��������ƫС��������Ӱ����)��
��2���ñ�NaOH��Һ�ζ�ʱ��Ӧ����NaOH��Һע��___�У���������������___������ͼ��ѡ������������������
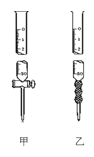
��3�����в���������ʵ����ƫ����ǣ�___�����ţ�
A.����ƿװҺǰ��������������ˮ
B.�ζ�ǰ����ʽ�ζ��ܼ��������ݣ��ζ���������
C.��ƿ��������ˮϴ�Ӻ����ô���Һ��ϴ
D.����ʽ�ζ�����ȡ����Һʱ��һ�δ���Һ������ƿ��
��4���жϴﵽ�ζ��յ�������ǣ�___��
��5��������ʵ�����ݼ�¼��
�ζ����� | ���������mL�� | NaOH��Һ�Ķ�����mL�� | |
�ζ�ǰ | �ζ��� | ||
1 | 20.00 | 0.00 | 18.28 |
2 | 20.00 | 0.00 | 18.24 |
ͨ������ɵã�������Ũ��Ϊ��___mol��L-1������������2λС������
���𰸡��� ƫ�� �� ��ʽ�ζ��� BC ���������һ������������Һʱ����Һǡ������ɫ��Ϊ�ۺ�ɫ�����ɫ�����Ұ�����ڲ��ָ�ԭ������ɫ 0.18
��������
��1���ζ���������ˮϴ�������ϴ������ϴ�ζ��ܣ���ʹ��Һ��Ũ�ȼ�С��
��2��������������Ϊ������Һ��Ӧʢ���ڼ�ʽ�ζ��ܣ���ϵζ�����״�жϽ��
��3�����������Ա�Һ�����Ӱ�죬����c�����⣩=![]() ������������
������������
��4���ζ�����Һ����ɫ���dz��ɫʱ���Ұ����֮�ڲ��ٸı䣬����ζ��յ㣻
��5�����1��2��ƽ������V��NaOH�����ټ���������Ũ�ȡ�
��1���ζ���������ˮϴ����������������Һ��ϴ���ʢٲ���������ϴ����Һ��Ũ�ȼ�С�����ĵ�������ⶨ���ƫ��
�ʴ�Ϊ���٣� ƫ��
��2����������Ϊǿ����м��ԣ�������ʽ�ζ��ܲ���������Ӧ��Ӧ���ü�ʽ�ζ��ܣ�ͼ��Ϊ��ʽ�ζ��ܣ�ͼ��Ϊ��ʽ�ζ��ܣ�
�ʴ�Ϊ���ң���ʽ�ζ��ܣ�
��3��A������ƿװҺǰ��������������ˮ����Ӱ�����Һ�����ʵ��������Բ�Ӱ�����ı�Һ���������Ӱ��ζ��������A��ѡ��
B���ζ�ǰ����ʽ�ζ��ܼ��������ݣ��ζ��������ݣ����ĵı�Һ��������c�����⣩=![]() ��֪���ζ����ƫ�ߣ���Bѡ��
��֪���ζ����ƫ�ߣ���Bѡ��
C����ƿ��������ˮϴ�Ӻ����ô���Һ��ϴ���������Һ�����ʵ��������ı�Һ���ƫ�ⶨ����жϣ���Cѡ��
D������ʽ�ζ�����ȡ����Һʱ��һ�δ���Һ������ƿ�⣬����Һ���ƫС�����ĵı�Һ���٣��ζ����ƫ�ͣ���D��ѡ��
��ѡ��BC��
��4���ζ�ǰ��ҺΪ��ɫ���ﵽ�ζ��յ�ʱ����ƿ�е���Һ����ɫ���dz��ɫ���Ұ�����ڲ��ٱ仯��
�ʴ�Ϊ�����������һ������������Һʱ����Һǡ������ɫ��Ϊ�ۺ�ɫ�����ɫ�����Ұ�����ڲ��ָ�ԭ������ɫ ��
��5�����εζ����ĵ����Ϊ��18.28mL��18.24mL�����ı�Һƽ�����Ϊ��18.26mL��
����������ʵ���Ũ��Ϊ��![]() =0.18mol/L��
=0.18mol/L��
�ʴ�Ϊ��0.18��

 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д�����Ŀ������þ��Mg3N2���ڹ�ҵ�Ͼ��зdz��㷺��Ӧ�á�ij��ѧ��ȤС����þ�뵪����Ӧ�Ʊ� Mg3N2 �������й�ʵ�顣ʵ��װ��������ʾ�� �����ּ���װ������ȥ��

��֪���ٵ���þ������Ϊdz��ɫ��ĩ��������ˮ��Ӧ��
���������ƺ��Ȼ����ȡ�����ķ�Ӧ���ҷ��ȣ������������ٶȽϿ졣
���¶Ƚϸ�ʱ���������ƻ�ֽ����O2�ȡ�
�ش��������⣺
��1������ b ��������__________��д��װ�� A �з�����Ӧ�Ļ�ѧ����ʽ___________��
��2����������Ӧ��ʼ������������ A ���ƾ��ƣ�ԭ����__________________��
��3��װ�� C ��Ϊ��������������Һ�� ��������______________��
��4�����Է�������
�������� | ʵ������ | ����ԭ�� |
ȡ������Ʒ���Թ��У� ����������ˮ | �Թܵײ��й��岻������ݼ�����ζ��������� | ��Ӧ�Ļ�ѧ����ʽΪ______________________ |
��ȥ�ϲ�[Һ�� ��������ϡ���� | �۲쵽����ȫ���ܽ⣬ ��������ð�� | ����ð����ԭ��Ϊ________________ |
����Ŀ������Ҫ��ش������й����⡣
��1���״���һ����Ҫ�Ļ���ԭ�ϣ���ҵ������CO2��H2��һ�������·�Ӧ�ϳɼ״�����֪��
��2CH3OH(l)+3O2(g)=2CO2(g)+4H2O(g) ��H1=-1275.6kJ��mol-1
��2CO(g)+O2(g)=2CO2(g) ��H2=-566.0kJ��mol-1
��H2O(g)=H2O(l) ��H3=-44.0kJ��mol-1
д���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ��___��
��2����¯������ұ��������Ҫ��������������Ҫ��ӦΪ��Fe2O3(s)+3CO(g)![]() 2Fe(s)+3CO2(g) H=��28.5kJ/mol��ұ������Ӧ��ƽ�ⳣ������ʽK=__���¶����ߺ�Kֵ__����������������������������С������
2Fe(s)+3CO2(g) H=��28.5kJ/mol��ұ������Ӧ��ƽ�ⳣ������ʽK=__���¶����ߺ�Kֵ__����������������������������С������
��3����T��ʱ����ӦFe2O3(s)+3CO(g)![]() 2Fe(s)+3CO2(g)��ƽ�ⳣ��K=64����2L�����ܱ������У����±���ʾ�������ʣ�����һ��ʱ���ﵽƽ�⡣
2Fe(s)+3CO2(g)��ƽ�ⳣ��K=64����2L�����ܱ������У����±���ʾ�������ʣ�����һ��ʱ���ﵽƽ�⡣
Fe2O3 | CO | Fe | CO2 | |
ʼ̬mol | 1.0 | 1.0 | 1.0 | 1.0 |
��ƽ��ʱCO ��ת����Ϊ___��
�����������־��Ӧ�ﵽƽ��״̬����__������ĸ����
a.�����������ܶȱ��ֲ���
b.����������ѹǿ���ֲ���
c.CO���������ʺ�CO2�������������
d.�����������ƽ����Է����������ֲ���