题目内容
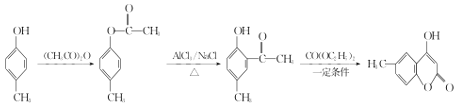
【题目】有机物J是一种防止血管中血栓形成与发展的药物,其合成路线如图所示(部分反应条件略去)。
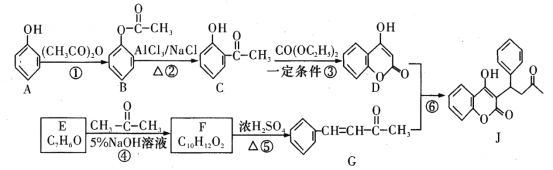
请回答下列问题:
(1)B的名称为______________,反应⑥的反应类型是__________。
(2)J含有_______种官能团。F的结构简式是_____________。
(3)反应③的化学方程式为____________________________________________-。
(4)写出同时满足下列条件的F的同分异构体的结构简式:______________(至少写两种)。
①苯环上只有两个处于对位的取代基;
②1 mol该有机物能与含2 mol NaOH的溶液恰好完全反应。
(5)参照D的合成路线,设计一种以![]() 为原料制备
为原料制备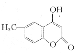 的合成路线__________。
的合成路线__________。
【答案】乙酸苯(酚)酯 加成反应 4 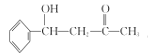

【解析】
物质A与乙酸酐反应,将酚羟基上的氢换成了乙酰基,在催化剂作用下,进行结构转化,此时酚羟基恢复,乙酰基进入酚羟基邻位; C到D的转化所有信息题已给,不必纠结如何反应,书写时关注原子守恒,判断出产物有乙醇即可。
(1)将B水解可得乙酸和苯酚,由系统命名法,B的名称为乙酸苯酯,G断开碳碳双键与D合成J,反应⑥的类型为加成反应;
(2)J中含有羟基、羰基、羧基、碳碳双键共4种官能团,根据F的分子式,据G的结构简式逆推可得F的结构简式为: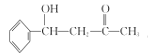 ;
;
(3)根据原子守恒和题中所给的反应物与部分生成物,可写出反应③的化学方程式为: ;
;
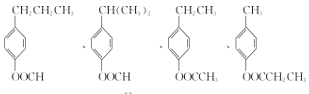
(4) 1 mol该有机物能与含2 mol NaOH的溶液恰好完全反应,且苯环上只有两个处于对位的取代基,则满足条件的同分异构体无法是酚,F中除去苯环还有一个不饱和度,可与氢氧化钠反应的物质只能是酯类,要反应掉2mol氢氧化钠,则应是酚酯,满足条件的同分异构体的结构简式有: ;
;
(5) 原料对比A多一个甲基,要合成的物质对比D多一个甲基,参考A合成D的方法可得: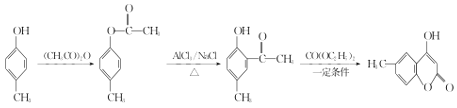 。
。

【题目】硫及其化合物有许多用途,相关物质的物理常数如下表所示:
H2S | S8 | FeS2 | SO2 | SO3 | H2SO4 | |
熔点/℃ | 85.5 | 115.2 | >600(分解) | 75.5 | 16.8 | 10.3 |
沸点/℃ | 60.3 | 444.6 | 10.0 | 45.0 | 337.0 |
回答下列问题:
(1)基态O原子的电子排布图为_______,基态O原子电子占据最高能级的电子云轮廓图为____形,O原子最外层电子的运动状态有_____种。
(2)根据价层电子对互斥理论,H2S、SO2、SO3的气态分子中,中心原子价层电子对数不同于其他分子的是______________。
(3)图(a)为S8的结构,该分子中S原子的杂化轨道类型为______________。其熔点和沸点要比二氧化硫的熔点和沸点高很多,主要原因为_____________。
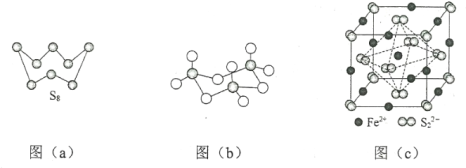
(4)气态三氧化硫以单分子形式存在,其分子中共价键的类型有_______种(根据成键的方式);固体三氧化硫中存在如图(b)所示的三聚分子,该分子中S原子的价层电子数___________。
(5)FeS2晶体的晶胞如图(c)所示。晶胞中有Fe2+_____个,有S22-_____个,晶胞中Fe2+位于S22-所形成的_____的体心。
(6)酸性H2SO4 __________H2SO3(填 “>”或 “<”)。
【题目】某研究小组为探究SO2和Fe(NO3)3溶液的反应的实质。设计了如下图所示装置进行实验.
已知:1.0 mol/L的Fe(NO3)3溶液的pH=1。

请回答:
(1)装置A中用于添加浓硫酸的仪器名称为__________________。
(2)实验前鼓入N2的目的是________________________________。
(3)装置B中产生了白色沉淀,其成分是________,说明SO2具有________性。
(4)分析B中产生白色沉淀的原因。
观点1:SO2与Fe3+反应;
观点2:在酸性条件下SO2与NO3-反应;
①若观点1正确,除产生沉淀外,还应观察到的现象是_________________。
②按观点2,装置B中反应的离子方程式是______________________________,
③有人认为,如将装置B中的Fe(NO3)3溶液替换为等体积的下列溶液,在相同条件下进行实验,也可验证观点2是否正确。此时应选择的最佳试剂是(填序号)_____。
A.1 mol/L稀硝酸 |
B.1.5 mol/L Fe(NO3)2溶液 |
C.6.0 mol/L NaNO3溶液和0.2 mol/L盐酸等体积混合的溶液 |
D.3.0 mol/L NaNO3溶液和0.1mol/L硫酸等体积混合的溶液 |