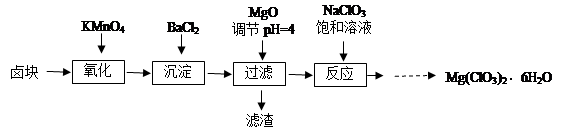��Ŀ����
ij��ѧʵ���Ҳ����ķ�Һ�к���Fe3����Cu2����Ba2����Cl���������ӣ���������з����Է�Һ���д������Ի��ս������Ʊ��Ȼ������Ȼ������塣
��1������1�к��еĽ��������� ��
��2������ʱ����H2O2��Һ������Ӧ�����ӷ���ʽΪ ��
��3�����������У�������Ϊ�Լ�X���� ������ĸ����
| A��BaCl2 | B��BaCO3 |
| C��NaOH | D��Ba(OH)2 |
��5���Ʊ��Ȼ�������������豣�������������Ŀ���� ��
��6���ɹ���2�õ�����Һ�Ʊ�BaCl2��ʵ���������Ϊ ����ȴ�ᾧ�� ��ϴ�ӡ����
��1������ͭ��2�֣���1�֣�
��2��2Fe2����2H����H2O2��2Fe3����2H2O��2�֣�
��3��BD��2�֣���1�֣�
��4��ȡ���һ��ϴ��Һ����������1��2����������Һ���������ְ�ɫ���ǣ�������ϴ����ȫ������ȡ���һ��ϴ��Һ����������1��2����������Һ���������ְ�ɫ���ǣ�������ϴ����ȫ������2�֣�
��5������Fe3��ˮ�⣨�����𰸾��ɣ���2�֣�
��6������Ũ�� ���ˣ�2�֣���1�֣�
���������������1��������м�ܰ�ͭ�û�������ͬʱ����ʣ�࣬����Ϊ����ͭ����2�����������������������Ϊ�����Ӻ��ٳ�����ȥ��˫��ˮ����Fe2������3������X�Լ���Ϊ�˵���pHʹ������������������������Ϊ�˲����������ʿ��Լ���BaCO3��Ba(OH)2�����ʣ���4���������ϴ���Ƿ�ɾ�һ��Ӧȡ���ε���Һ������ܺ��е��������ӣ�һ�������������ӡ������ӵȣ���5���Ȼ�����ˮ��Һ��ˮ�⣬�����������������Fe3��ˮ�⣻��6�����˺�õ�����ҺΪϡ��Һ������Һ�еõ�������Բ�������Ũ�����½ᾧ�ķ�����
���㣺���鹤ҵ�����в���ԭ�������й����⡣

��(Sr)������������Ԫ�أ��䵥�ʺͻ�����Ļ�ѧ������ơ��������ơ�ʵ�����ú�̼���ȵķ���(��SrCO3 38.40%��SrO12.62%��CaCO3 38.27%��BaCO3 2.54%���������������������8.17%)�Ʊ������ȴ�Ʒ�IJ���ʵ��������£�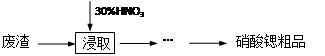
��1������Ũ�������������Ϊ65%���ܶ�Ϊ1.4g/cm3��Ҫ����30%ϡ����500mL������Ҫ���ĵ������� �������ƹ����в�ʹ����ƽ�������Ҫ����������� ������Ҫʹ�õ������� ��
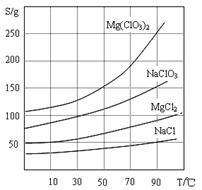
��֪�����ε��ܽ��(g/100 gˮ)���±�
| �¶�/������ | 0 | 20 | 30 | 45 | 60 | 80 | 100 |
| Sr(NO3)2 | 28.2 | 40.7 | 47 | 47.2 | 48.3 | 49.2 | 50.7 |
| Ca(NO3)2��4H2O | 102 | 129 | 152 | 230 | 300 | 358 | 408 |
��2���ɽ�ȡ��õ��Ļ�����Ʊ������ȴ�Ʒ��ʵ�鲽������Ϊ�����ˡ� �� ��ϴ�ӣ����
��֪��������������л��ܼ�A�С�ʽ����Sr(NO3)2�C212��Ba(NO3)2�C261��Ca(NO3)2�C164
��3���Ƶõ������ȴ�Ʒ�к�����Ca(NO3)2��Ba(NO3)2�����ʡ��ⶨ�����ȴ��ȵ�ʵ�����£���ȡ5.39g��������Ʒ�������������л��ܼ�A�������ˡ�ϴ�ӡ������ʣ�����5.26g�����˹������250 mL����Һ��ȡ��25.00 mL������pHΪ7������ָʾ������Ũ��Ϊ0.107mol/L��̼������Һ�ζ����յ㣬����̼������Һ22.98mL��
�ζ����̵ķ�Ӧ��Sr2����CO32���� SrCO3������Ba2����CO32���� BaCO3��
�ٵζ�ѡ�õ�ָʾ��Ϊ ���ζ��յ�۲쵽������Ϊ ��
�ڸ������ȴ�Ʒ�У������ȵ���������Ϊ ��С���������λ�������ζ�ǰ��Ʒ��Ca(NO3)2û�г��������ⶨ�������ȴ��Ƚ��� (�ƫ�ߡ�����ƫ�͡����䡱)��
�����̿��Ʊ�������ص���Ҫ��Ӧ���£�
�������� 3MnO2+KClO3+6KOH 3K2MnO4+KCl+3H2O
3K2MnO4+KCl+3H2O
�����绯 3K2MnO4+2CO2=2KMnO4+MnO2��+2K2CO3
������ʵ��ܽ�ȣ�293K�����±���
| | K2CO3 | KHCO3 | K2SO4 | KMnO4 |
| �ܽ��/g | 111 | 33.7 | 11.1 | 6.34 |
a�������� b�������� c�������� d��������
��2������ʱ�����������ԭ���� �������������ԭ���� ��
��3�����õ�ⷨҲ����ʵ��K2MnO4��ת����2K2MnO4+2H2O
 2KMnO4+2KOH+H2������ԭ������ȣ���ⷨ������Ϊ ��
2KMnO4+2KOH+H2������ԭ������ȣ���ⷨ������Ϊ ����4�������Ƶζ�������������ش��Ȳ������£�
�� ��ȡ0.80 g���ҵĸ�����ز�Ʒ�����50 mL��Һ��
�� ȷ��ȡ0.2014 g�����Ѻ�ɵ�Na2C2O4��������ƿ�У�������������ˮʹ���ܽ⣬�ټ������������ữ��
�� ��ƿ����Һ���ȵ�75��80 �棬�����â������Ƶĸ��������Һ�ζ����յ㡣��¼���ĸ��������Һ�����������ó���Ʒ���ȡ�
�ٵζ������з�Ӧ�����ӷ���ʽΪ ��
�ڴﵽ�ζ��յ�ı�־Ϊ ��
�ۼ����¶ȴ���90�棬���ֲ��ᷢ���ֽ⣬�ᵼ�²�ò�Ʒ���� �����ƫ�ߡ�����ƫ�͡�����Ӱ�족��
�ܽ�һ�������������Һ���ữ�IJ�������Һ��ϣ���÷�ӦҺ��Mn2+��Ũ���淴Ӧʱ��t�ı仯��ͼ����ԭ�����Ϊ ��

�±����������ڲ�ͬ�¶��µ��ܽ�ȣ�g/100gˮ����
| | NaNO3 | KNO3 | NaCl | KCl |
| 10�� | 80.5 | 21.2 | 35.7 | 31.0 |
| 100�� | 175 | 246 | 39.1 | 56.6 |
�����裺�����ʱ��Ӱ����Ե��ܽ�ȣ����뾧��ʱ���ܼ�����ĺ��Բ��ƣ�
ijͬѧ��������ʵ���֮��Ϊ1��1�������ƺ��Ȼ���Ϊԭ�ϣ�����һ������ˮ��ȡ����ص�ʵ�飬����������ͼ��ʾ��

��1���ڢٺ͢ڵ�ʵ������У���Ҫ���ƵĹؼ���ʵ��������______________________�������������У�______���A����C����ӦΪ����ؾ��塣
��2���ڢٵ�ʵ������У���Ҫ���еIJ���������________________��________________��_____________��
��3���ֲ�Ʒ�п��ܺ�����������_______________________����������һ�����ӵķ�����________________________________________________________________________��
��4��Ϊ��ϴ�����õ�����ؾ��壬�����ܼ�������ϴ�Ӽ�����___________�����ţ���
a����ˮ b����ˮ c��95%�ľƾ� d�����Ȼ�̼
��5����ȡ34.0g�����ƺ�29.8g�Ȼ��أ�����70gˮ����100��������50gˮ��ά�ָ��¶ȣ����ˣ��������������Ϊ_______________��