��Ŀ����
��ͼΪ������ļס����������أ�
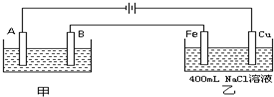
�Իش�
��1�����׳����õ��ԭ�������϶�������A��
��2�����׳���������43.2g���ҳ���������һֱ����������������ҳ��������Ϸų��������ڱ�״���µ������

�Իش�
��1�����׳����õ��ԭ�������϶�������A��
Fe
Fe
������
����
��������缫���Ϻ͵缫���ƣ����缫��Ӧ��Ag++e-=Ag
Ag++e-=Ag
��B��Ag
Ag
������
����
��������缫���Ϻ͵缫���ƣ����缫��Ӧ��Ag-e-=Ag+
Ag-e-=Ag+
��Ӧѡ�õĵ������Һ��AgNO3
AgNO3
����2�����׳���������43.2g���ҳ���������һֱ����������������ҳ��������Ϸų��������ڱ�״���µ������
4.48
4.48
L����������1����ͼ��֪��A���Դ������������AΪ�������׳����õ��ԭ�������϶���������Ӧ���������������������Һ�к��������ӣ��Դ���������
��2�����õ����غ��֪2Ag��2e-��H2�����Դ����������������ʵ������ټ��������ڱ�״���µ������
��2�����õ����غ��֪2Ag��2e-��H2�����Դ����������������ʵ������ټ��������ڱ�״���µ������
����⣺��1����ͼ��֪��A���Դ������������AΪ�����������϶������������������缫��ӦΪAg++e-=Ag��
�����������缫��ӦΪAg-e-=Ag+���ҵ��Һ�к��������ӣ���ѡAgNO3�����Һ��
�ʴ�Ϊ��Fe��������Ag++e-=Ag��Ag��������Ag-e-=Ag+��AgNO3��
��2���׳���������Ag++e-=Ag���ҳ�������2H++e-=H2����
Ag�����ʵ���Ϊ
=0.4mol��
�ɵ����غ��֪��2Ag��2e-��H2�������������ʵ���Ϊ0.2mol��
�ڱ�״���µ����Ϊ0.2mol��22.4L/mol=4.48L���ʴ�Ϊ��4.48��
�����������缫��ӦΪAg-e-=Ag+���ҵ��Һ�к��������ӣ���ѡAgNO3�����Һ��
�ʴ�Ϊ��Fe��������Ag++e-=Ag��Ag��������Ag-e-=Ag+��AgNO3��
��2���׳���������Ag++e-=Ag���ҳ�������2H++e-=H2����
Ag�����ʵ���Ϊ
| 43.2g |
| 108g/mol |
�ɵ����غ��֪��2Ag��2e-��H2�������������ʵ���Ϊ0.2mol��
�ڱ�״���µ����Ϊ0.2mol��22.4L/mol=4.48L���ʴ�Ϊ��4.48��
���������⿼����ԭ�������õ����غ�ļ��㣬��ȷ���������жϼ������ĵ缫��Ӧ�ǽ����Ĺؼ����ѶȲ���

��ϰ��ϵ�д�
 ��ҵ����ϵ�д�
��ҵ����ϵ�д� ͬ��ѧ��һ�ζ���ϵ�д�
ͬ��ѧ��һ�ζ���ϵ�д� �����ܾ�ϵ�д�
�����ܾ�ϵ�д�
�����Ŀ
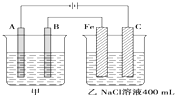 ��ͼΪ������ļ����������أ���ش�
��ͼΪ������ļ����������أ���ش�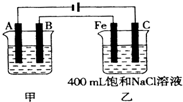 ��ͼΪ������ļס��������أ��Իش�
��ͼΪ������ļס��������أ��Իش�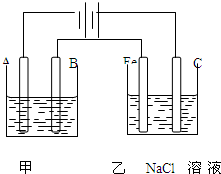 ��ͼΪ������ļ����������أ���ش�
��ͼΪ������ļ����������أ���ش�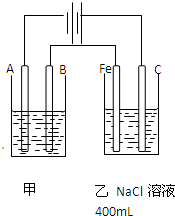 ��ͼΪ������ļ����������أ���ش�
��ͼΪ������ļ����������أ���ش�