��Ŀ����
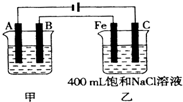 ��ͼΪ������ļס��������أ��Իش�
��ͼΪ������ļס��������أ��Իش���1�����������õ��ԭ�������϶�������A��
��
��
������
����
����缫���Ϻ͵缫���ƣ����缫��Ӧ��Ag++e-�TAg
Ag++e-�TAg
��B��Ҫ��ͬA������
��
������
����
���缫��Ӧ��
Ag-e-�TAg+
Ag-e-�TAg+
��Ӧѡ�õĵ������Һ����������Һ
��������Һ
����2���ҵ�����������������̪��Һ����ʼ���һ��ʱ�䣬����������
��
��
ɫ��C��������dz����
dz����
ɫ����3����������������4.32g�����Ҳ��������Ϸų��������ڱ�״���µ������
448
448
mL����4�����ҵ�����ʣ����Һ��Ϊ400mL�������������Һ�����������ʵ����ʵ���Ũ��Ϊ
0.1
0.1
mol/L����Һ��pH����13
13
����������1�����������õ��ԭ�������϶���������ǵ�Ƴأ��Ʋ����������Ƽ��������������ϵõ��ӷ�����ԭ��Ӧ��������ʧ���ӷ���������Ӧ��
��2�����ǵ��أ�̼�����������������������������ӷŵ磬�����������ӷŵ磻
��3������ת�Ƶ����غ�����������������
��4�������������������ƵĹ�ϵʽ�����������Ƶ����ʵ���Ũ�ȣ��ٸ���ˮ�����ӻ���������������Ũ�ȣ��Ӷ��ó���Һ��pH��
��2�����ǵ��أ�̼�����������������������������ӷŵ磬�����������ӷŵ磻
��3������ת�Ƶ����غ�����������������
��4�������������������ƵĹ�ϵʽ�����������Ƶ����ʵ���Ũ�ȣ��ٸ���ˮ�����ӻ���������������Ũ�ȣ��Ӷ��ó���Һ��pH��
����⣺��1���׳��ǵ�Ƴأ��������������������������������ӵõ��ӷ�����ԭ��Ӧ���缫��ӦʽΪAg++e-�TAg����������ʧ���ӷ���������Ӧ���缫��ӦʽΪAg-e-�TAg+���������ҺΪ�����Ե���������Һ��
�ʴ�Ϊ������������Ag++e-�TAg������������Ag-e-�TAg+��AgNO3��Һ��
��2�����ǵ��أ�̼�缫�������ӷŵ��������������缫�������ӷŵ�����������ͬʱ��Һ���������������ӣ��������缫������Һ�ʼ��ԣ���̪�Լ�������ɫ���������缫������Һ���ɫ��̼�缫������������������dz����ɫ���壬����ˮ��ʹ��Һ��dz����ɫ������C�缫������dz����ɫ��
�ʴ�Ϊ���죻dz����ɫ��
��3���׳�������������������������������4.32g��ת�Ƶ��ӵ����ʵ���=1��
=0.04mol��
�ҳ�����������������������1mol����ת�Ƶ��ӵ����ʵ�����2mol��
��ת��0.04mol����ʱ�������������ʵ���Ϊ0.02mol��
���������=0.02mol��22.4L/mol=0.224L=448mL��
�ʴ�Ϊ��448��
��4���ҳ��е�ط�ӦʽΪ2NaCl+2H2O
H2��+Cl2��+2NaOH�������������������ƵĹ�ϵʽ֪������0.02mol����ʱͬʱ����0.04mol�������ƣ�
���������Ƶ����ʵ���Ũ��=
=0.1mol/L��
����Һ��������Ũ��=
mol/L=10-13 mol/L��
������pH=13��
�ʴ�Ϊ��0.1��13��
�ʴ�Ϊ������������Ag++e-�TAg������������Ag-e-�TAg+��AgNO3��Һ��
��2�����ǵ��أ�̼�缫�������ӷŵ��������������缫�������ӷŵ�����������ͬʱ��Һ���������������ӣ��������缫������Һ�ʼ��ԣ���̪�Լ�������ɫ���������缫������Һ���ɫ��̼�缫������������������dz����ɫ���壬����ˮ��ʹ��Һ��dz����ɫ������C�缫������dz����ɫ��
�ʴ�Ϊ���죻dz����ɫ��
��3���׳�������������������������������4.32g��ת�Ƶ��ӵ����ʵ���=1��
| 4.32g |
| 108g/mol |
�ҳ�����������������������1mol����ת�Ƶ��ӵ����ʵ�����2mol��
��ת��0.04mol����ʱ�������������ʵ���Ϊ0.02mol��
���������=0.02mol��22.4L/mol=0.224L=448mL��
�ʴ�Ϊ��448��
��4���ҳ��е�ط�ӦʽΪ2NaCl+2H2O
| ||
���������Ƶ����ʵ���Ũ��=
| 0.04mol |
| 0.4L |
����Һ��������Ũ��=
| 10-14 |
| 0.1 |
������pH=13��
�ʴ�Ϊ��0.1��13��
���������⿼���˵���ԭ������ȷ���ӷŵ�˳���ǽⱾ��ؼ������ת�Ƶ����غ���н���Ѷ��еȣ�

��ϰ��ϵ�д�
 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
�����Ŀ
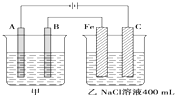 ��ͼΪ������ļ����������أ���ش�
��ͼΪ������ļ����������أ���ش�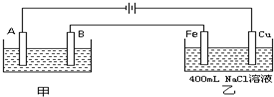
 ��ͼΪ������ļ����������أ���ش�
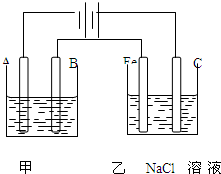
��ͼΪ������ļ����������أ���ش�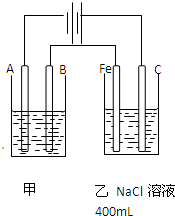 ��ͼΪ������ļ����������أ���ش�
��ͼΪ������ļ����������أ���ش�