��Ŀ����
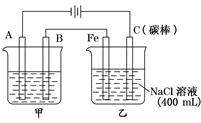
 ��ͼΪ������ļ����������أ���ش�
��ͼΪ������ļ����������أ���ش���1���׳���Ϊ�õ�⾫��ͭ��װ�ã�A����
����
����
����������ͭ
��ͭ
���缫��ӦΪCu2++2e-�TCu
Cu2++2e-�TCu
��B��������
����
����������ͭ
��ͭ
���缫��ӦΪCu-2e-�TCu2+
Cu-2e-�TCu2+
���������ҺΪCuSO4��Һ
CuSO4��Һ
����2�����ײ���������12.8g�����Ҳ������ų������ڱ�״���µ����Ϊ
4.48
4.48
L����������1����⾫����ͭʱ����ͭ����������ͭ���������������ҺΪ�����Ե�ͭ�Σ�
��2��������·��ת�Ƶ�����ȣ�����ת�Ƶ�����Ƚ��м��㣮
��2��������·��ת�Ƶ�����ȣ�����ת�Ƶ�����Ƚ��м��㣮
����⣺��1����⾫����ͭʱ����ͭ����������ͭ���������������ҺΪ�����Ե�ͭ�Σ�����ͼƬ֪��A��������B������������A�������Ǵ�ͭ��������ͭ���ӵõ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ��Cu2++2e-�TCu��
B���������缫�����Ǵ�ͭ��������ͭʧ���ӷ���������Ӧ���缫��ӦʽΪ��Cu-2e-�TCu2+��
�����һ���ÿ����Ե�����ͭ�ܣ�
�ʴ�Ϊ����������ͭ��Cu2++2e-�TCu����������ͭ��Cu-2e-�TCu2+��CuSO4��Һ��
��2��������·��ת�Ƶ�����ȣ����ײ���������12.8g���������ϵõ����ӵ����ʵ���=
��2=0.4mol���Ҳ��������������ӷŵ���������������ת�Ƶ�����ȵã����Ҳ������ų������ڱ�״���µ����=
��22.4L/mol=4.48L��
�ʴ�Ϊ��4.48��
B���������缫�����Ǵ�ͭ��������ͭʧ���ӷ���������Ӧ���缫��ӦʽΪ��Cu-2e-�TCu2+��
�����һ���ÿ����Ե�����ͭ�ܣ�
�ʴ�Ϊ����������ͭ��Cu2++2e-�TCu����������ͭ��Cu-2e-�TCu2+��CuSO4��Һ��
��2��������·��ת�Ƶ�����ȣ����ײ���������12.8g���������ϵõ����ӵ����ʵ���=
| 12.8g |
| 64g/mol |
| 0.4mol |
| 2 |
�ʴ�Ϊ��4.48��
���������⿼���˵���ԭ������ȷ�������Ϸ����ķ�Ӧ�ǽⱾ��ؼ����ٽ��ת�Ƶ�����ȷ�������ѶȲ���

��ϰ��ϵ�д�
 ��ҵ����ϵ�д�
��ҵ����ϵ�д� ͬ��ѧ��һ�ζ���ϵ�д�
ͬ��ѧ��һ�ζ���ϵ�д�
�����Ŀ
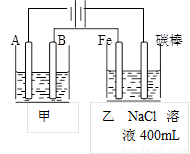
 ��ͼΪ������ļ����������أ���ش�
��ͼΪ������ļ����������أ���ش�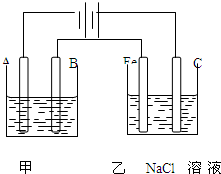 ��ͼΪ������ļ����������أ���ش�
��ͼΪ������ļ����������أ���ش�