��Ŀ����
�����ڼ���ȼ�ű��ڻ����������SO2�������ߣ���ɴ�����Ⱦ��ijʵ��С��ͬѧ��̽��SO2�����ʣ����ⶨ������SO2�ĺ�����
��1�������������ʵ��װ�ã��������̽�������ش����⣺
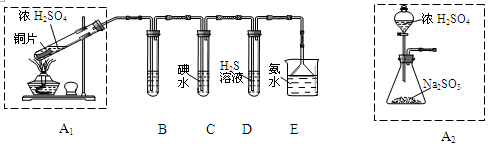
��װ��A1�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��װ��B���ڼ���SO2��Ư���ԣ�������ʢ�Լ�Ϊ ��װ��D���ڼ���SO2�� ���ʣ�
��װ��C�з�Ӧ�����ӷ���ʽΪ ��
��Ϊ��ʵ����ɫ������Ŀ�꣬��ͬѧ����װ��A2����װ��A1������Ϊװ��A2���ŵ��ǣ�д���㣩 �� ��
��2�������������·����ⶨ������SO2�����������������������ԭ�����壩��
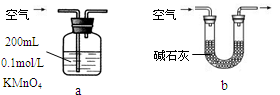
������Ϊ�ĸ�װ�ÿ��У�����ţ� ��ʹ������ѡ�õ�װ�òⶨSO2����ʱ������Ҫ�ⶨ���������� ��
������Ϊ�ĸ�װ�ò����У�����ţ� ��˵������ ��
��1�������������ʵ��װ�ã��������̽�������ش����⣺

��װ��A1�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��װ��B���ڼ���SO2��Ư���ԣ�������ʢ�Լ�Ϊ ��װ��D���ڼ���SO2�� ���ʣ�
��װ��C�з�Ӧ�����ӷ���ʽΪ ��
��Ϊ��ʵ����ɫ������Ŀ�꣬��ͬѧ����װ��A2����װ��A1������Ϊװ��A2���ŵ��ǣ�д���㣩 �� ��
��2�������������·����ⶨ������SO2�����������������������ԭ�����壩��

������Ϊ�ĸ�װ�ÿ��У�����ţ� ��ʹ������ѡ�õ�װ�òⶨSO2����ʱ������Ҫ�ⶨ���������� ��
������Ϊ�ĸ�װ�ò����У�����ţ� ��˵������ ��
��1���� Cu + 2H2SO4(Ũ)  CuSO4 + 2H2O + SO2������Ʒ����Һ����������SO2 + I2 + 2H2O ��SO42- + 2I��+ 4H+ �� �ܲ��ü��ȣ���Լ��Դ����ԼҩƷ�������ȫ�����ڿ��Ʒ�Ӧ���У���Ӧ����֣� ��2���� a ����KMnO4��Һ����ɫʱ���ⶨͨ����������V�� �� b �������к��е�CO2Ҳ�����ʯ�ҷ�Ӧ����ɲ�����ȷ��
CuSO4 + 2H2O + SO2������Ʒ����Һ����������SO2 + I2 + 2H2O ��SO42- + 2I��+ 4H+ �� �ܲ��ü��ȣ���Լ��Դ����ԼҩƷ�������ȫ�����ڿ��Ʒ�Ӧ���У���Ӧ����֣� ��2���� a ����KMnO4��Һ����ɫʱ���ⶨͨ����������V�� �� b �������к��е�CO2Ҳ�����ʯ�ҷ�Ӧ����ɲ�����ȷ��
 CuSO4 + 2H2O + SO2������Ʒ����Һ����������SO2 + I2 + 2H2O ��SO42- + 2I��+ 4H+ �� �ܲ��ü��ȣ���Լ��Դ����ԼҩƷ�������ȫ�����ڿ��Ʒ�Ӧ���У���Ӧ����֣� ��2���� a ����KMnO4��Һ����ɫʱ���ⶨͨ����������V�� �� b �������к��е�CO2Ҳ�����ʯ�ҷ�Ӧ����ɲ�����ȷ��
CuSO4 + 2H2O + SO2������Ʒ����Һ����������SO2 + I2 + 2H2O ��SO42- + 2I��+ 4H+ �� �ܲ��ü��ȣ���Լ��Դ����ԼҩƷ�������ȫ�����ڿ��Ʒ�Ӧ���У���Ӧ����֣� ��2���� a ����KMnO4��Һ����ɫʱ���ⶨͨ����������V�� �� b �������к��е�CO2Ҳ�����ʯ�ҷ�Ӧ����ɲ�����ȷ�������������1������װ��A1��Cu��Ũ���Ṳ�ȷ�����Ӧ��Cu + 2H2SO4(Ũ)
 CuSO4 + 2H2O + SO2������װ��B���ڼ���SO2��Ư���ԣ�SO2����ijЩ��ɫ������Ʒ�����γ���ɫ�����ʣ����SO2��Ư���ԡ���װ��B��Ʒ����Һ���顣��װ��D�з�����Ӧ��SO2+2H2S=3S��+H2O��SO2�������������������ԣ�H2S�ǻ�ԭ�������ֻ�ԭ�ԡ�����װ��C��SO2���ˮ����I2��SO2��2H2O=H2SO4��2HI����Ӧ�����ӷ���ʽΪ��I2��SO2��2H2O= SO42��+4H++2I-����Ϊ��ʵ����ɫ������Ŀ�꣬��ͬѧ����װ��A2����װ��A1��ʹ��װ��A2���ŵ��Dz��ü��ȣ����Խ�Լ��Դ�����ȫ�����ڿ��Ʒ�Ӧ���У���Ӧ����ֵȣ���2���� ������SO2�Ŀ���ͨ�뵽KMnO4��Һ�У�ֻ��SO2�ܷ�����Ӧ�����Կ��Բⶨ������SO2��������ʹ�����ַ���ֻ��ⶨ��KMnO4��Һ����ɫʱ���ⶨͨ����������V���ɡ�����b�����ڿ����к��е�CO2Ҳ�����ʯ�ҷ�Ӧ��ˮ����Ҳ�ܱ����գ���˻���ɲ�����ȷ���������ڲⶨ������SO2������2����ȡ�����顢���ʡ��ⶨ����ѧ����ʽ�����ӷ���ʽ����д��֪ʶ��
CuSO4 + 2H2O + SO2������װ��B���ڼ���SO2��Ư���ԣ�SO2����ijЩ��ɫ������Ʒ�����γ���ɫ�����ʣ����SO2��Ư���ԡ���װ��B��Ʒ����Һ���顣��װ��D�з�����Ӧ��SO2+2H2S=3S��+H2O��SO2�������������������ԣ�H2S�ǻ�ԭ�������ֻ�ԭ�ԡ�����װ��C��SO2���ˮ����I2��SO2��2H2O=H2SO4��2HI����Ӧ�����ӷ���ʽΪ��I2��SO2��2H2O= SO42��+4H++2I-����Ϊ��ʵ����ɫ������Ŀ�꣬��ͬѧ����װ��A2����װ��A1��ʹ��װ��A2���ŵ��Dz��ü��ȣ����Խ�Լ��Դ�����ȫ�����ڿ��Ʒ�Ӧ���У���Ӧ����ֵȣ���2���� ������SO2�Ŀ���ͨ�뵽KMnO4��Һ�У�ֻ��SO2�ܷ�����Ӧ�����Կ��Բⶨ������SO2��������ʹ�����ַ���ֻ��ⶨ��KMnO4��Һ����ɫʱ���ⶨͨ����������V���ɡ�����b�����ڿ����к��е�CO2Ҳ�����ʯ�ҷ�Ӧ��ˮ����Ҳ�ܱ����գ���˻���ɲ�����ȷ���������ڲⶨ������SO2������2����ȡ�����顢���ʡ��ⶨ����ѧ����ʽ�����ӷ���ʽ����д��֪ʶ��
��ϰ��ϵ�д�
�����Ŀ