��Ŀ����
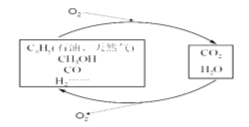
����Ŀ����-���Ȼ�ѧѭ������ʾ��ͼ���£�
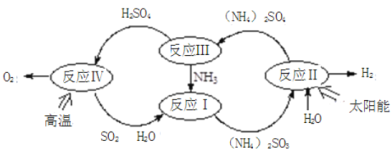
��1����Ӧ���ǽ�̫����ת��Ϊ���ܣ���ת��Ϊ��ѧ�ܣ����������ĵ缫��Ӧʽ _______________;
��2����Ӧ���п��Ʒ�Ӧ��������Ҫ����ͬ����������立ֽ���ﲻͬ������װ��A-B-C����������ԣ���ͼʾ���¼����Լ���ͨ��N2�ž���������400������װ��A ��(NH4)2SO4��ȫ�ֽ������ֹͣ���ȣ���ȴ��ֹͣͨ��N2���۲쵽װ��A��B֮��ĵ���������������ɫ���塣�����飬�ð�ɫ�����װ��B����Һ����SO32-����SO42-����һ���о����֣�����������������
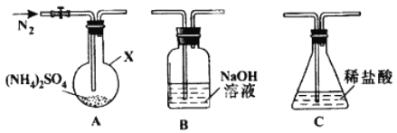
(NH4)2 SO4��400���ֽ�Ļ�ѧ����ʽ��:________________________________________;
��3����Ӧ����2H2SO4(l)=2SO2(g)+O2(g)+2H2O (l) ��H=+462kJ/mol
����������Ӧ��ɣ�i��H2SO4(l)=SO3(g)+H2O(g) ��H=+177kJ/mol
ii��SO3(g)�ֽ⡣
iii.H2O(l)=H2O(g) ��H=+44kJ/mol
��L(L1��L2)��X�ɷֱ����ѹǿ���¶ȡ���ͼ��ʾ Lһ��ʱ��ii��SO3(g)��ƽ��ת������X�ı仯��ϵ��
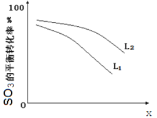
i��X����������������________________,
ii���ж�L1��L2�Ĵ�С��ϵ�����������ɣ�________________________________________;
����SO3�ֽ���Ȼ�ѧ����ʽΪ��__________________________________________________;
��4�������ܱ������У����Ʋ�ͬ�¶Ƚ���SO3�ֽ�ʵ�顣SO3��ʼŨ�Ⱦ�Ϊ cmol��L��1���ⶨSO3��ת���ʣ������ͼ��ͼ��������ΪSO3��ƽ��ת�������¶ȵĹ�ϵ�������߱�ʾ��ͬ�¶��·�Ӧ������ͬ��Ӧʱ����δ�ﵽ��ѧƽ��ʱSO3��ת���ʡ�
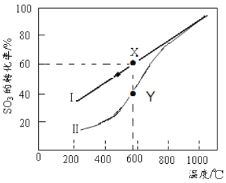
��ͼ�е�X��ƽ�ⳣ����K=_____ ��
��Y���Ӧ�¶��µķ�Ӧ���ʣ�v(��)______v(��)(ѡ�����������)�����¶ȵ����ߣ������߱ƽ������ߵ�ԭ���ǣ�_____________________________________��
���𰸡�
��1��SO32--2e-+H2O=SO42-+2H+
��2��3(NH4)2SO4 ![]() 4NH3��+ N2��+3SO2��+ 6H2O��
4NH3��+ N2��+3SO2��+ 6H2O��
��3����ѹǿ L1��L2 SO3�ֽ���������ѹǿ��ͬ������������¶ȵ�������SO3��ת��������
��2SO3(g)![]() 2SO2(g)+O2(g) ��H=+196kJ/mol
2SO2(g)+O2(g) ��H=+196kJ/mol
��4�� ��0.675c �� �� �¶����ߣ���Ӧ���ʼӿ죬�ﵽƽ������Ҫ��ʱ������
��������
�����������1�����ص���������������Ӧ���缫��ӦʽΪSO32--2e-+H2O=SO42-+2H+��
��2���������400��ʱ�ֽ⣬�����ˮ�������A��B��C�������壬A�ǿ����к������ĵ��ʣ���Ϊ������B��ʹʪ��ĺ�ɫʯ����ֽ������Ϊ������C��ʹƷ����Һ��ɫ��Ϊ��������Ӧ�Ļ�ѧ����ʽΪ3(NH4)2SO4![]() 4NH3��+N2��+3SO2��+6H2O����
4NH3��+N2��+3SO2��+6H2O����
��3����i����ͼ��֪��XԽ��ת����Խ�ͣ������¶�ת����������X��ʾѹǿ���ʴ�Ϊ��ѹǿ��
ii����2SO3(g)=2SO2(g)+O2(g)��H��0���¶ȸߣ�ת���ʴ�ͼ�е�ѹǿʱL2��Ӧ��ת���ʴ���L1��L2��
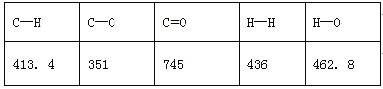
����֪��ӦiiΪ2SO3(g)=2SO2(g)+O2(g)�����ݸ�˹���ɷ�Ӧ����2H2SO4(l)=2SO2(g)+O2(g)+2H2O(l) ���Կ���i��2+ii-iii��2����SO3�ֽ���Ӧ����H=(462-177��2+44��2)kJ/mol =+196kJ/mol����SO3�ֽ���Ȼ�ѧ����ʽΪ2SO3(g)![]() 2SO2(g)+O2(g) ��H=+196kJ/mol��
2SO2(g)+O2(g) ��H=+196kJ/mol��
��4�� �� 2SO3(g)![]() 2SO2(g)+O2(g)
2SO2(g)+O2(g)
��ʼŨ��(mol��L��1) c 0 0
�仯Ũ��(mol��L��1) 0.6c 0.6c 0.3c
ƽ��Ũ��(mol��L��1) 0.4c 0.6c 0.3c
��ʱƽ�ⳣ��K=[(0.6c)2��0.3c]��(0.4c)2=0.675c��
��Y��ʱ��Ӧ��û�дﵽƽ��״̬��������У���������Ӧ���ʴ����淴Ӧ����;�¶ȵ����ߣ�����b������a�ƽ�����Ӧ���ʼӿ죬�ﵽƽ��ʱ��ʱ�����̡�