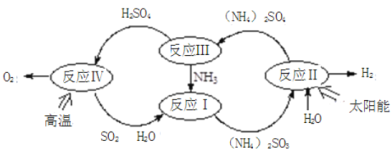
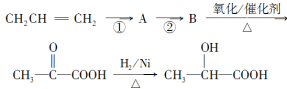��Ŀ����
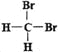
����Ŀ��ij����ֻ��C��H��O����Ԫ�أ������ģ����ͼ��ʾ�������й���12��ԭ��(ͼ��������֮������ߴ���������˫���Ȼ�ѧ��)��
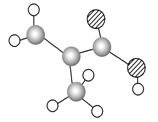
��1�������ʵĽṹ��ʽΪ________________��
��2�������������������ŵ�����Ϊ______________��
��3�����������У���ò�Ʒ��Ϊͬϵ�����(�����)__________����Ϊͬ���칹�����____________��
��CH3CH===CHCOOH ��CH2===CHCOOCH3 ��CH3CH2CH===CHCOOH
��CH3CH(CH3)COOH ��CH2===CHCOOH
��4����������һ�������·����Ӿ۷�Ӧ�Ļ�ѧ����ʽΪ__________________________��
���𰸡���1����![]() ��
��
��2��̼̼˫�����Ȼ�
��3�� ����������������
��4��CH2=CH(CH3)COOH ![]()
��������
�����������1���ɷ���ģ�Ϳ�֪���л���Ľṹ��ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��2���ɣ�1���нṹ��ʽ��֪���л����к���̼̼˫�����Ȼ������ţ��ʴ�Ϊ��̼̼˫�����Ȼ���
��3�����л���Ľṹ��ʽΪ![]() ����֮��Ϊͬϵ�����CH3CH2CH�TCHCOOH��CH2�TCHCOOH����ֻ��Ϊͬ���칹�����CH3CH�TCHCOOH��CH2�TCHCOOCH3���ʴ�Ϊ��������������������4�������ʷ����Ӿ۷�Ӧ�ķ���ʽΪ��CH2=CH(CH3)COOH
����֮��Ϊͬϵ�����CH3CH2CH�TCHCOOH��CH2�TCHCOOH����ֻ��Ϊͬ���칹�����CH3CH�TCHCOOH��CH2�TCHCOOCH3���ʴ�Ϊ��������������������4�������ʷ����Ӿ۷�Ӧ�ķ���ʽΪ��CH2=CH(CH3)COOH ![]()
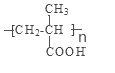 ��
��

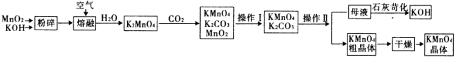
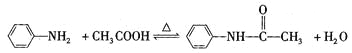
����Ŀ������������һ�ְ�ɫ�й���Ƭ״�ᾧ���ɫ�ᾧ��ĩ���ǻǰ���ҩ���ԭ�ϣ�������ֹʹ�������ȼ��ͷ������������������Ʊ�ԭ��Ϊ
ʵ�����
���� | ��Է� | ��״ | �ܶ�(g/mL) | �е�( | �ܽ��� | |
���� | 93 | ��ɫ��״Һ�� | 1.02 | 184.4 | ����ˮ | �������Ҵ������� |
���� | 60 | ��ɫҺ�� | 1.05 | 118.1 | ������ˮ | �������Ҵ������� |
���� | 135 | ��ɫ���� | 1.22 | 304 | ������ˮ����������ˮ | �������Ҵ������� |
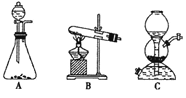
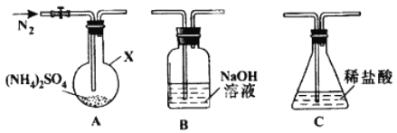
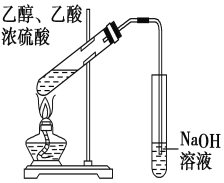
ʵ��װ����ͼ��ʾ�������������г�װ������ȥ����
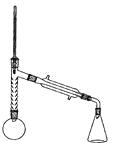
ע�������ͷ������������൱�ڶ����������ڷе���̫��Ļ����ķ��롣�������ױ�������
ʵ�鲽�裺
����1����100mLԲ����ƿ�м�����ˮ����9.3mL��������18.4mL��п��0.1g��������ʯ����װ��������Բ����ƿ���ȼ��ȣ�ʹ��ӦҺ����״̬�»��������ڼ����¶ȣ�ʹ�¶ȼ��¶ȿ�����105![]() ���ң���ӦԼ60~80min����Ӧ���ɵ�ˮ���������ᱻ����������Ӧ�������ʱ��ֹͣ���ȡ�
���ң���ӦԼ60~80min����Ӧ���ɵ�ˮ���������ᱻ����������Ӧ�������ʱ��ֹͣ���ȡ�
����2���ڽ����£����Ƚ�������ƿ�е�Һ����ϸ��״����ʢ�б�ˮ��100mL�ձ��У����ٽ��裬���������ᾧ�������ձ����Լ���ȴ�����º��г��ˡ�ϴ�ӡ�����ɵõ�����������Ʒ��
����3��������������Ʒ�����ؽᾧ�����ᾧ��ȫ����ˣ�����ѹ�ʸ��˱�������Ʒ���ڸɾ��ı����������ɣ����أ�������ʡ�
��ش��������⣺
��1��ʵ���м���п�۵�Ŀ����_____________________��
��2���ӻ�ѧƽ��ĽǶȷ����������¶ȼƵ��¶���105![]() ���ҵ�ԭ����______________��
���ҵ�ԭ����______________��
��3��ͨ��_____________���жϷ�Ӧ������ɡ�
��4������1���ȿ���_____________������ˮԡ��������ԡ������
��5��ϴ������������Ʒ����ʵķ�����__________________������ĸ����
A����������ˮϴ
B����������ˮϴ
C��������ˮϴ��������ˮϴ
D���þƾ�ϴ
��6������2�õ��Ĵ�Ʒ������ijЩ���ʶ���ɫ�������3�ؽᾧ�IJ����ǣ�����Ʒ����ˮ�ܽ⣬�������̿0.1g����н�����ɫ��______________���ٽ���Һ��ȴ�ᾧ��
��7����ʵ�����յõ���Ʒ9.1g�������������IJ�����____________��С���������λ���֡���