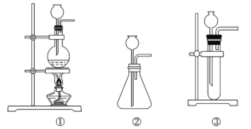
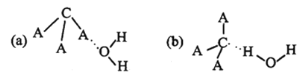��Ŀ����
����Ŀ����1���ڱ�״���£�ȡ�ס��ҡ�����30.0mL��ͬŨ�ȵ����ᣬȻ��ֱ��������������ͬ��þ���Ͻ��ĩ�����±����й����ݣ����跴Ӧǰ����Һ����������仯������ش�
�ټ���ʵ���У����ᣨѡ������������������������������������ͬ��___������ʵ��������___��
����������ʵ���Ũ��Ϊ___��
�ۺϽ���Mg��Al�����ʵ���֮��Ϊ___��
��2���ס������ձ��и�ʢ1L0.6mol/L�������NaOH��Һ�������ձ��зֱ��������������ۣ���Ӧ�����������ɵ�����������V(�ף���V���ң�=3��4����������۵�����Ϊ___��
���𰸡����� ������ 1.00mol/L 1��1 7.2g
��������
��1�����кϽ������С���ң������������ҲС���ң�˵�����кϽ������������ǹ����ġ������������ɵ�����������ͬ�������кϽ�����������ң�˵�����кϽ���������������ġ�
��2�������NaOH��Һ�ֱ������Ӧ��2Al+6HCl=2AlCl3+3H2����2Al+2NaOH+2H2O=2NaAlO2+3H2�����������������ɵ�������HCl��NaOH������������HCl��NaOH���ɵ������������Ϊ1:3������ͼ���������ɵ��������������������������������ȡ���������������������Ƚ�������֮�䣬������������ķ�Ӧ�У����������ڼ�����ķ�Ӧ�У��������
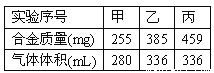
��1���ٸ�������ķ���������ʵ���У������ǹ����ģ����ںϽ��������ͬ�ģ������ռ���ı�����255g�Ͻ�������280mL���壬������ʵ��������Ҳ�������ģ���385g�Ͻ�������423mL���壬��ʵ�����������������ֻ��336mL�����������������Dz������ġ�
�ڱ����������������ģ������е���ȫ���������ᣬ336mL����µ���������ʵ���Ϊ0.015mol������HCl�����ʵ���Ϊ0.03mol����������ʵ���Ũ��Ϊ1.00mol/L��
�ۼ����кϽ�ȫ����Ӧ���������������ʵ���Ϊ0.0125mol����þ�����ʵ���Ϊx���������ʵ���Ϊy������з����飺![]() �����
�����![]() ���ʺϽ���Mg��Al�����ʵ���֮��Ϊ1��1��
���ʺϽ���Mg��Al�����ʵ���֮��Ϊ1��1��
��2�������Ϸ�����֪����������ķ�Ӧ�У����������ڼ�����ķ�Ӧ�У��������n(HCl)=n(NaOH)=0.6mol����2Al+6HCl=2AlCl3+3H2���У�0.6molHCl��������0.3mol���������ɵ�����������V(�ף���V���ң�=3��4����ͬ�����£�����ȵ������ʵ���֮�ȣ�������NaOH������Ӧ�������������ʵ���Ϊ0.4mol�����ݷ�Ӧ2Al+2NaOH+2H2O=2NaAlO2+3H2�����������۵����ʵ���Ϊ![]() mol������Ϊ
mol������Ϊ![]() mol��27g/mol=7.2g��
mol��27g/mol=7.2g��