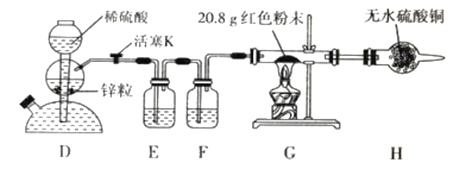
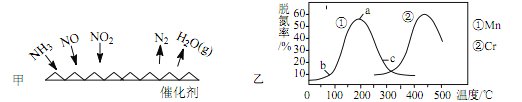��Ŀ����
����Ŀ��������������(FeC2O4��2H2O)��һ�ֵ���ɫ��ĩ��ij����С����������װ�ü�����������������ȷֽ�IJ��ֲ��
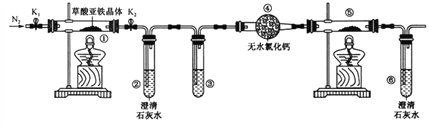
����˵����ȷ����
A. ���ۺ͢��зֱ�ʢ������NaOH��Һ��CuO���ɼ������ɵ�CO
B. ʵ��ʱֻ��Ҫ��װ�â��з�Ӧ��������ͨ��N2
C. �������е���ˮCaCl2������ˮ����ͭ�ɼ���ֽ����ɵ�ˮ����
D. ʵ��������е���ɫ��ĩ��ȫ��ɺ�ɫ�������һ��Ϊ��
���𰸡�A
��������A�������ڡ�����ȥCO2�����е���ˮ�Ȼ��ƽ����������������CuO����ת��ɺ�ɫ����Ӧһ������CO�����A��ȷ��B��ʵ�鿪ʼ��װ���еĿ����Էֽ⼰���鶼�и��ţ����Ա�����ͨ��N2��ȥװ���еĿ�����B����C�����ڴ��ڡ�����Һ�е�������������ˮ������������з�����ˮ����ͭ������ֽ����ɵ�ˮ������C����D��������������ֽ�ʣ��Ĺ���ΪFeO�����û����ȫ��Ϊ��ɫ��Ҳ�п��������ھ���û����ȫ�ֽ⣬D������ȷ��A��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�������������ڹ�ũҵ�����ж�����ҪӦ�á�
��1���£�N2H4���������ᷴӦ�����ɵ�����һ���⻯��ڱ�״���£����⻯��������ܶ�Ϊ1.92g/L�������е�Ԫ�ص���������Ϊ0.977����÷�Ӧ�Ļ�ѧ����ʽΪ____.
��2������������һ�������£����Է������·�Ӧ��SO2(g)+NO2(g)![]() SO3(g)+NO(g) ��H= -42kJ��mol-1����2L�ĺ����ܱ������г���SO2(g)��NO2(g)����ʵ���������£�
SO3(g)+NO(g) ��H= -42kJ��mol-1����2L�ĺ����ܱ������г���SO2(g)��NO2(g)����ʵ���������£�
ʵ���� | �¶� | ��ʼ���ʵ���mol | ƽ��ʱ���ʵ���/mol | |
n(SO2) | n(NO2) | n(NO) | ||
�� | T1 | 4.0 | 1.0 | 0.9 |
�� | T2 | 1.0 | 4.0 | 0.8 |
�� | T2 | 0.4 | 0.6 | a |
����ʵ����У���2minʱ��÷ų���������8.4kJ��0��2minʱ���ڣ���SO2��ʾ��ƽ����Ӧ����v(SO2)=_______________�����¶��µ�ƽ�ⳣ��Ϊ_________�������������С�������λ��
���ɱ������ݿ���֪��T1_______T2������>����<������=����
��ʵ����У��ﵽƽ��ʱNO2��ת����Ϊ______________.
��3����ҵ�Ͽ����ð�ˮ��SO2ת��ΪNH4HSO3����������(NH4)2SO4�������²��NH4HSO3��ҺpHΪ6������Һ��![]() ________________________.����֪��H2SO3��Kal =1.5��10-2Ka2 =1.0��10-7��
________________________.����֪��H2SO3��Kal =1.5��10-2Ka2 =1.0��10-7��
��4��ʹ�ü�ӵ绯ѧ�����Դ���ú�����е�NO��װ����ͼ����֪������Һ��pH��4��7֮�䣬д�������ĵ缫��Ӧʽ__________________��
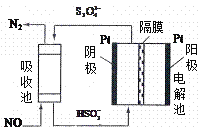 ��
��