��Ŀ����
����Ŀ�������̼����(Na2CS3)������ɱ��������������ijС�����ʵ��̽�������̼���Ƶ����ʲ��ⶨ����Һ��Ũ�ȡ�
ʵ��1��̽��Na2CS3������
���� | ���������� |
�� | ȡ����Na2CS3������������ˮ���Ƴ���Һ���ֳ����ȷ� |
�� | ������һ����Һ�еμӼ��η�̪��Һ����Һ���ɫ |
�� | ����һ����Һ�еμ�����KMnO4��Һ����ɫ��ȥ |
��1��H2CS3��___��(��ǿ����)��
��2����֪����۵�����������SO42-��д���÷�Ӧ�����ӷ���ʽ___��
ʵ��2���ⶨNa2CS3��Һ��Ũ�ȣ�����ͼ��ʾ���Ӻ�װ�ã�ȡ100mLNa2CS3��Һ����������ƿ�У�������d�Ļ�������������2.0mol/LϡH2SO4���رջ�����
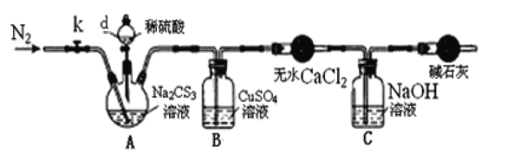
��֪��Na2CS3+H2SO4=Na2SO4+CS2+H2S����CS2��H2S���ж���CS2������ˮ���е�46�棬��CO2ijЩ�������ƣ���NaOH��������Na2COS2��H2O��
��3��ʢ����ˮCaCl2��������������___��
��4����Ӧ���������k���ٻ���ͨ����N2һ��ʱ�䣬��Ŀ����___��
��5��Ϊ�˼���Na2CS3��Һ��Ũ�ȣ���B�л������й��ˡ�ϴ�ӡ�������أ���19.2g���壬��A��Na2CS3�����ʵ���Ũ��Ϊ___��(����1λС��)
���𰸡��� 5CS32-+24MnO4-+52H+=5CO2��+15SO42-+24Mn2++26H2O (����)����� ��װ���в�����H2S��CS2ȫ���������װ���У�ʹ�䱻��ȫ���� 2.0mol/L
��������
(1)Na2CS3��ˮ��Һ�м����̪���ɫ��˵��Na2CS3��ǿ�������Σ�
(2)����۵�����������SO42������ԭ������Mn2����ͬʱ������CO2��H2O��
(3)A�з���Na2CS3+H2SO4=Na2SO4+CS2+H2S�������ɵ��������B������ͭ��Ӧ����CuS��������ˮ�Ȼ�������ˮ������������ͼ���ж����������ƣ�
(4)��Ӧ���������k���ٻ���ͨ����N2һ��ʱ�䣬������ȫ��������װ�����գ�
(5) ��B�л������й��ˡ�ϴ�ӡ�������أ��õ�19.2g��ɫ���������ݷ�Ӧ����ʽ������ϵʽΪ![]() ���ó������
�������
(1)![]() ��ˮ��Һ�м����̪���ɫ��˵��
��ˮ��Һ�м����̪���ɫ��˵��![]() ��ǿ�������Σ���
��ǿ�������Σ���![]() Ϊ���ᣬ
Ϊ���ᣬ
�ʴ�Ϊ������
(2)����![]() ������������
������������![]() ����ԭ�����������ӣ�ͬʱ�����ɶ�����̼��ˮ�����ӷ���ʽΪ
����ԭ�����������ӣ�ͬʱ�����ɶ�����̼��ˮ�����ӷ���ʽΪ![]() ��
��
�ʴ�Ϊ��![]() ��
��
(3)ʢ����ˮ![]() ������Ϊ
������Ϊ![]() ����
����![]() ����ܣ�
����ܣ�
�ʴ�Ϊ��![]() ����
����![]() ����ܣ�
����ܣ�
(4)��Ӧ���������k���ٻ���ͨ����![]() һ��ʱ�䣬��Ŀ���ǣ���װ���е�
һ��ʱ�䣬��Ŀ���ǣ���װ���е�![]() ȫ������B�б�������գ���װ���е�
ȫ������B�б�������գ���װ���е�![]() ȫ������C�б�������գ�
ȫ������C�б�������գ�
�ʴ�Ϊ����װ���в�����![]() ��
��![]() ȫ���������װ���У�ʹ�䱻��ȫ���գ�
ȫ���������װ���У�ʹ�䱻��ȫ���գ�
(5)��A�з�Ӧ��ȫ��K����ͨ����![]() һ��ʱ�䣬Ȼ���B�л������й��ˡ�ϴ�ӡ�������أ���
һ��ʱ�䣬Ȼ���B�л������й��ˡ�ϴ�ӡ�������أ���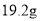 ��ɫ���壬
��ɫ���壬![]() �����ݹ�ϵʽ
�����ݹ�ϵʽ![]() ��
��![]() ��
��![]() ��
��
�ʴ�Ϊ��![]() ��
��

����Ŀ������ʵ������淶���ܴﵽʵ��Ŀ�ĵ���
ʵ����� | ʵ��Ŀ�� | |
A | ��һ�������£������������ϩ��������ͨ������ | ��ȥ�����е���ϩ |
B | �ò�����պȡ����Һ�����ڸ����pH��ֽ�в���Ƭ�̺������ɫ���Ƚ϶��� | ���ԲⶨNaClO��ҺpH |
C | ��0.1 mol/LMgSO4��Һ���뵽2mlNaOH��Һ���������г��������ٵ���0.1 mol/LCuSO4��Һ | �Ƚ�Mg��OH��2��Cu��OH��2��Ksp��С |
D | ������������Һ�еμ����ᱵ������ | ��������������Һ�Ƿ���� |
A.AB.BC.CD.D