��Ŀ����
����Ŀ����Ҫ����д���пո�
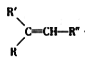
(1)CH��CCH(CH3)2ϵͳ����������Ϊ_____________��
(2)֧��ֻ��һ���һ���ʽ����С�������Ľṹ��ʽΪ __________��
(3)2��3��������1����ϩ�Ľṹ��ʽΪ_________________��
(4)ij������ʽΪ C6H14����������������Ȳ���������ӳɵõ���������Ľṹ��ʽΪ______��
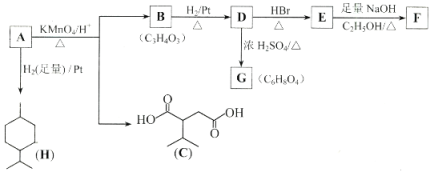
(5)����A~E���������ӵ�ʾ��ͼ�ش����⡣
��B������������������ __________________��
����������������C��ͬϵ����� _____________(�����)��
�۵����ʵ���������������ȫȼ��ʱ���� O2������_________������������������ȫȼ��ʱ���� O2������______________(�����)��
���𰸡�3-��-1-��Ȳ 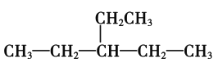 CH2=C(CH3)CH(CH3)2 (CH3)2CHCH(CH3)2 ̼̼˫�� A D A
CH2=C(CH3)CH(CH3)2 (CH3)2CHCH(CH3)2 ̼̼˫�� A D A
��������
(1)Ȳ������ʱ��Ҫѡ�������ŵ����̼��Ϊ��������������������һ�˸������ϵ�̼ԭ�ӱ�ţ�����ʾ�������ŵ�λ�ã�
(2)�����к���ȡ�����һ������������ٺ���5��Cԭ�ӣ��ݴ�д�����������������Ľṹ��ʽ��
(3)���л���Ϊϩ��������ϩ����ϵͳ�������γ���ṹ��ʽ��
(4)��������������Ȳ���������ӳɵõ���������⣬������C�Ϻ�2��H��
(5)AΪ��������ģ�ͣ�BΪ��ϩ�����ģ�ͣ�CΪ����Ľṹ��ʽ��DΪ�������ģ�ͣ�EΪ��������ģ�ͣ������л���Ľṹ�����ʽ��
(1)ѡ�����̼��Ϊ������������������һ�˿�ʼ��ţ�1��̼����һ��̼̼������3��̼��һ�����������Ϊ4��̼������ϵͳ�����������л��������Ϊ3-��-1-��Ȳ��
(2)֧����һ���һ����һ����ܹ����������˵�ǰ����̼ԭ���ϣ���ʽ����С��������������������5��̼ԭ�ӣ����л��������Ϊ3-�һ����飬�ṹ��ʽΪ�� ��
��
(3)��������2��3��������1����ϩ����������4��̼ԭ�ӣ�1��̼����һ��̼̼˫����2��3��̼ԭ���Ϸֱ���һ����������л���Ľṹ��ʽΪCH2=C(CH3)CH(CH3)2��
(4)ij������ʽΪC6H14����������������Ȳ���������ӳɵõ���������⣬������C�Ϻ�2��H�����������Ľṹ��ʽΪ(CH3)2CHCH(CH3)2��
(5)AΪ��������ģ�ͣ�BΪ��ϩ�����ģ�ͣ�CΪ����Ľṹ��ʽ��DΪ�������ģ�ͣ�EΪ��������ģ�ͣ�
��BΪ��ϩ�����ģ�ͣ���ϩ����ϩ����������Ϊ̼̼˫����
�ڽṹ���ơ������ͬ���ڷ�����������һ��������-CH2-��ԭ���ŵ��л��ﻥΪͬϵ�CΪ���飬AΪ���飬���������ͬ���ṹ���ƣ��ڷ�����������������-CH2-��ԭ���ţ�����Ϊͬϵ�
�������ʵ���ʱ�����ʷ����к��е�C��Hԭ����Խ�࣬����O2��Խ�ࡣA��ʾ����CH4��B��ʾ��CH2=CH2��C��ʾ����C3H8�� D��ʾ��C6H6�� E��ʾ����C3H8������������ʵ����ʵ�������1mol��1mol CH4��ȫȼ������O2�����ʵ�����2mol��1mol��ϩ��ȫȼ������O2�����ʵ�����3mol��1mo|������ȫȼ������O2�����ʵ�����5mol��1mol����ȫȼ������O2�����ʵ�����7.5mol���ɼ������ʵ���������������ȫȼ������O2�����ʵ��������DZ�����ѡD��
����������ʱ����������1mol O2��4mol Hԭ����������4g��ͬ������1mol O2����1molCԭ�ӣ���������12g��������12g����HԪ��Ҫ����3molO2��CԪ������1molO2�����Ե������IJ�ͬ�����л�����HԪ�صĺ���Խ�ߣ�����O2��Խ�࣬����CH4��HԪ�غ�����࣬���Ե�����ʱ��ȫȼ��ʱ����O2������CH4����ѡA��

 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д� ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�����Ŀ�������̼����(Na2CS3)������ɱ��������������ijС�����ʵ��̽�������̼���Ƶ����ʲ��ⶨ����Һ��Ũ�ȡ�
ʵ��1��̽��Na2CS3������
���� | ���������� |
�� | ȡ����Na2CS3������������ˮ���Ƴ���Һ���ֳ����ȷ� |
�� | ������һ����Һ�еμӼ��η�̪��Һ����Һ���ɫ |
�� | ����һ����Һ�еμ�����KMnO4��Һ����ɫ��ȥ |
��1��H2CS3��___��(��ǿ����)��
��2����֪����۵�����������SO42-��д���÷�Ӧ�����ӷ���ʽ___��
ʵ��2���ⶨNa2CS3��Һ��Ũ�ȣ�����ͼ��ʾ���Ӻ�װ�ã�ȡ100mLNa2CS3��Һ����������ƿ�У�������d�Ļ�������������2.0mol/LϡH2SO4���رջ�����
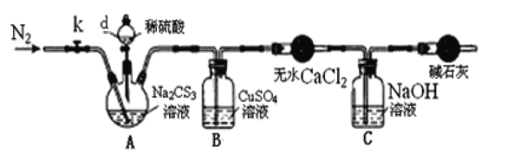
��֪��Na2CS3+H2SO4=Na2SO4+CS2+H2S����CS2��H2S���ж���CS2������ˮ���е�46�棬��CO2ijЩ�������ƣ���NaOH��������Na2COS2��H2O��
��3��ʢ����ˮCaCl2��������������___��
��4����Ӧ���������k���ٻ���ͨ����N2һ��ʱ�䣬��Ŀ����___��
��5��Ϊ�˼���Na2CS3��Һ��Ũ�ȣ���B�л������й��ˡ�ϴ�ӡ�������أ���19.2g���壬��A��Na2CS3�����ʵ���Ũ��Ϊ___��(����1λС��)