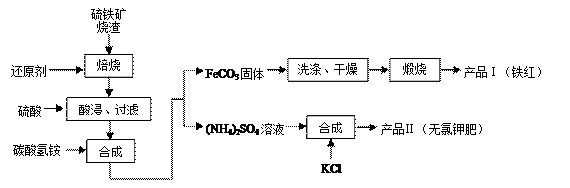
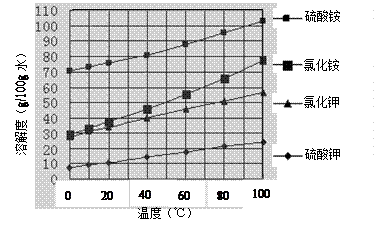
��Ŀ����
��12�֣��ҹ��зḻ�ĺ�ˮ��Դ�����������ú�ˮ��Դ�ǵ�ǰ��ѧ �о���һ����Ҫ����
�о���һ����Ҫ����
��1������������Ԫ�ص��� �� ��дԪ�ط��ţ���
��2����ҵ�ϳ���ʳ��Ϊԭ���Ʊ����������������Ʊ�Ư�ۣ���д��Ư�۳���¶���ڿ�����ʧЧ�ķ�Ӧ�Ļ�ѧ����ʽ �� ��
��3���Ӻ�ˮ����ȡʳ�κ���Ĺ������£�
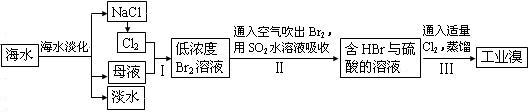
�ٲ�������ѻ��Br2����������ֽ�Br2��ԭΪBr¯����Ŀ��Ϊ������Ԫ�أ���д�������Ļ�ѧ����ʽ�� �� ��
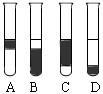 ����3mL��ˮ�У�����1mL���Ȼ�̼�������ú۲쵽�Թ���ķֲ�����Ϊ��ͼ�� �� ��
����3mL��ˮ�У�����1mL���Ȼ�̼�������ú۲쵽�Թ���ķֲ�����Ϊ��ͼ�� �� ��
��ij��ѧ�о���ѧϰС��Ϊ�˽�ӹ�ҵ�����ᴿ��ķ������������й����ϣ�Br2�ķе�Ϊ59�档����ˮ���ж��Ժ�ǿ��ʴ�ԡ����Dzι��������̺����������װ�ü�ͼ��
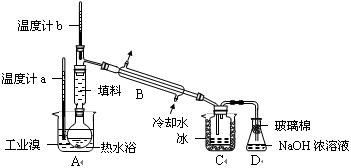
 �������������ۣ�
�������������ۣ�
��ʵ��װ�����������ã�Ҫ��C�л�ô�����Һ�弴�ﵽ�ᴿ���Ŀ�ģ������п��ƵĹؼ������ǣ� �� ��
�� Ϊ��ȥ�ò������Բ���������Cl2���������м��� �� ��Һ����ַ�Ӧ���ٽ��еķ�������� �� ��
Ϊ��ȥ�ò������Բ���������Cl2���������м��� �� ��Һ����ַ�Ӧ���ٽ��еķ�������� �� ��
 �о���һ����Ҫ����
�о���һ����Ҫ������1������������Ԫ�ص��� �� ��дԪ�ط��ţ���
��2����ҵ�ϳ���ʳ��Ϊԭ���Ʊ����������������Ʊ�Ư�ۣ���д��Ư�۳���¶���ڿ�����ʧЧ�ķ�Ӧ�Ļ�ѧ����ʽ �� ��
��3���Ӻ�ˮ����ȡʳ�κ���Ĺ������£�

�ٲ�������ѻ��Br2����������ֽ�Br2��ԭΪBr¯����Ŀ��Ϊ������Ԫ�أ���д�������Ļ�ѧ����ʽ�� �� ��
 ����3mL��ˮ�У�����1mL���Ȼ�̼�������ú۲쵽�Թ���ķֲ�����Ϊ��ͼ�� �� ��
����3mL��ˮ�У�����1mL���Ȼ�̼�������ú۲쵽�Թ���ķֲ�����Ϊ��ͼ�� �� ����ij��ѧ�о���ѧϰС��Ϊ�˽�ӹ�ҵ�����ᴿ��ķ������������й����ϣ�Br2�ķе�Ϊ59�档����ˮ���ж��Ժ�ǿ��ʴ�ԡ����Dzι��������̺����������װ�ü�ͼ��


 �������������ۣ�
�������������ۣ���ʵ��װ�����������ã�Ҫ��C�л�ô�����Һ�弴�ﵽ�ᴿ���Ŀ�ģ������п��ƵĹؼ������ǣ� �� ��
��
 Ϊ��ȥ�ò������Բ���������Cl2���������м��� �� ��Һ����ַ�Ӧ���ٽ��еķ�������� �� ��
Ϊ��ȥ�ò������Բ���������Cl2���������м��� �� ��Һ����ַ�Ӧ���ٽ��еķ�������� �� ��
��

��ϰ��ϵ�д�
 ͬ������ϵ�д�
ͬ������ϵ�д�
�����Ŀ
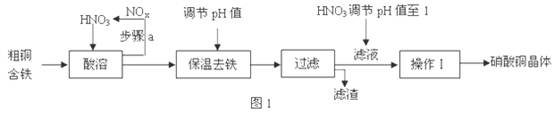
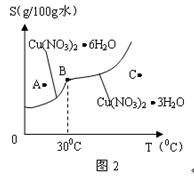
 2Fe��Al2O3.���ֽ���ұ���ķ�������
2Fe��Al2O3.���ֽ���ұ���ķ������� ��
��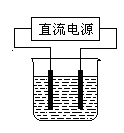
 2H2����O2�����������Ǹ�ԭ�������������Һ��С�������� g��
2H2����O2�����������Ǹ�ԭ�������������Һ��С�������� g��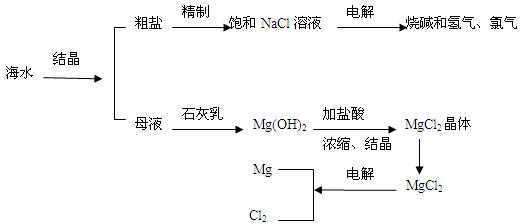
 MgO + H2O
MgO + H2O  Mg + Cl2��
Mg + Cl2�� �����ͻ�������β�����к������ŷ�
�����ͻ�������β�����к������ŷ�