��Ŀ����
��ϩ��ʯ���ѽ�������Ҫ�ɷ֣������ͨ����������һ������ʯ�ͻ�����չˮƽ���ش��������⣺
��1����ϩ�Ľṹ��ʽΪ ��
��2�������ܼ���������ϩ���Լ��� ��������ţ�
A��ϡ���� B��������Ȼ�̼��Һ C��ˮ D�����Ը��������Һ
��3��������ϩΪ��Ҫԭ�Ϻϳ����ᣬ��ϳ���·��ͼ��ʾ
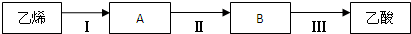
��B���ʹ����ŵ����� ��
�ڷ�Ӧ��Ļ�ѧ����ʽΪ ����Ӧ������ ��
�۹�ҵ������ϩΪԭ�Ͽ��Ժϳ�һ����Ҫ���л��߷��ӻ�����÷�Ӧ�Ļ�ѧ����ʽΪ ��
��1����ϩ�Ľṹ��ʽΪ
��2�������ܼ���������ϩ���Լ���
A��ϡ���� B��������Ȼ�̼��Һ C��ˮ D�����Ը��������Һ
��3��������ϩΪ��Ҫԭ�Ϻϳ����ᣬ��ϳ���·��ͼ��ʾ
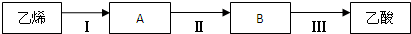
��B���ʹ����ŵ�����
�ڷ�Ӧ��Ļ�ѧ����ʽΪ
�۹�ҵ������ϩΪԭ�Ͽ��Ժϳ�һ����Ҫ���л��߷��ӻ�����÷�Ӧ�Ļ�ѧ����ʽΪ
���㣺��ϩ�Ļ�ѧ����
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�
��������1����ϩ�����д���̼̼˫����
��2�������DZ������������ȶ�����ϩ����˫���������巢���ӳɷ�Ӧ���ܱ����Եĸ������������
��3����ϩ��ˮ�ӳ������Ҵ����Ҵ��������õ���ȩ����ȩ�����õ�����ݴ˽��
��BΪ��ȩ��������Ϊȩ����
���Ҵ�������������ȩ��
����ϩ�����Ӿ۷�Ӧ���ɾ���ϩ��
��2�������DZ������������ȶ�����ϩ����˫���������巢���ӳɷ�Ӧ���ܱ����Եĸ������������
��3����ϩ��ˮ�ӳ������Ҵ����Ҵ��������õ���ȩ����ȩ�����õ�����ݴ˽��
��BΪ��ȩ��������Ϊȩ����
���Ҵ�������������ȩ��
����ϩ�����Ӿ۷�Ӧ���ɾ���ϩ��
���
�⣺��1����ϩ�Ľṹ��ʽΪ��CH2=CH2��
�ʴ�Ϊ��CH2=CH2��
��2�������DZ������������ȶ������ܱ����Եĸ�����������������ᡢ�Ӧ����ϩ����̼̼˫�������ʻ��ã��ܹ����巢���ӳɷ�Ӧʹ������Ȼ�̼��ɫ���ܹ���ԭ���Եĸ������ʹ���������ɫ��
��ѡ��BD��
��3����ϩ��ˮ�ӳ������Ҵ����Ҵ��������õ���ȩ����ȩ�����õ�����
��B��������ȩ��������Ϊȩ����
�ʴ�Ϊ��ȩ����
���Ҵ�������������ȩ����Ӧ�Ļ�ѧ����ʽΪ��2CH3CH2OH+O2
2CH3CHO+2H2O��
�ʴ�Ϊ��2CH3CH2OH+O2
2CH3CHO+2H2O��������Ӧ��
����ϩ�����ӳɾۺϷ�Ӧ���ɾ���ϩ����Ӧ�ķ���ʽΪ��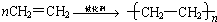 ��
��
�ʴ�Ϊ��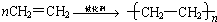 ��
��
�ʴ�Ϊ��CH2=CH2��
��2�������DZ������������ȶ������ܱ����Եĸ�����������������ᡢ�Ӧ����ϩ����̼̼˫�������ʻ��ã��ܹ����巢���ӳɷ�Ӧʹ������Ȼ�̼��ɫ���ܹ���ԭ���Եĸ������ʹ���������ɫ��
��ѡ��BD��
��3����ϩ��ˮ�ӳ������Ҵ����Ҵ��������õ���ȩ����ȩ�����õ�����
��B��������ȩ��������Ϊȩ����
�ʴ�Ϊ��ȩ����
���Ҵ�������������ȩ����Ӧ�Ļ�ѧ����ʽΪ��2CH3CH2OH+O2
| ͭ |
| �� |
�ʴ�Ϊ��2CH3CH2OH+O2
| ͭ |
| �� |
����ϩ�����ӳɾۺϷ�Ӧ���ɾ���ϩ����Ӧ�ķ���ʽΪ��
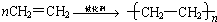 ��
���ʴ�Ϊ��
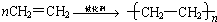 ��
��
���������⿼����ϩ�����ʣ���Ϥ��ϩ�Ľṹ�ǽ���ؼ�����Ŀ�ѶȲ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��0.2mol MnO2��50mL 12mol?L-1�����Ϻ����ȣ���Ӧ��ȫ�������µ���Һ�м���������AgNO3��Һ������AgCl�����ʵ���Ϊ������������Ļӷ�����������
| A������0.3 mol |
| B����0.3 mol |
| C������0.3��0.6 mol֮�� |
| D�����϶�����ȷ |
�������ʵ��۵�ߵ�˳����ȷ���ǣ�������
| A�����ʯ������裾̼���� |
| B��K��Na��Li |
| C��NaF��NaCl��NaBr |
| D��CI4��CBr4��CCl4��CH4 |
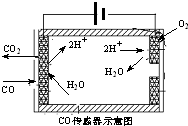 �������ִ������а�����Խ��Խ��Ҫ�Ľ�ɫ������β����̼�⻯����������Pһ����̼�ȣ������Ļ�����ȾԽ��Խ���ԣ������������ŷ��ѳ�Ϊ���д�����Ⱦ����Ҫ��Դ��
�������ִ������а�����Խ��Խ��Ҫ�Ľ�ɫ������β����̼�⻯����������Pһ����̼�ȣ������Ļ�����ȾԽ��Խ���ԣ������������ŷ��ѳ�Ϊ���д�����Ⱦ����Ҫ��Դ��