��Ŀ����
7�� 25��ʱ������ƽ�ⳣ����
25��ʱ������ƽ�ⳣ����| ���ữѧʽ | CH3COOH | HClO | H2CO3 |
| ����ƽ�ⳣ����25�棩 | 1.75��10-5 | 3.0��10-8 | K1=4.4��10-7 K2=4.7��10-11 |
��1�����ʵ���Ũ��Ϊ0.1mol��L-1������4�����ʣ�pH�ɴ�С��˳����A��B��D��C��
A��Na2CO3
B��NaClO
C��CH3COONa
D��NaHCO3
��2����������ˮ�д������Ũ�ȣ�������ˮ�м����������BD��
A��Na2CO3
B��NaClO
C��CH3COONa
D��NaHCO3
��3��������0.1mol��L-1��CH3COOH��Һ��ˮϡ���̣����б���ʽ������һ����С����A��
A��c��H+�� B��$\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$ C��c��H+����c��OH-�� D��$\frac{c��O{H}^{-}��}{c��{H}^{+}��}$
��4�����Ϊ10mLpH=2�Ĵ�����Һ��һԪ��HX�ֱ��ˮϡ����1000mL��ϡ����pH�仯����ͼ��ʾ����HX�ĵ���ƽ�ⳣ�����ڣ�����ڡ������ڡ���С�ڡ��������ƽ�ⳣ����ϡ�ͺ�HX��Һ��ˮ���������c��H+�����ڴ�����Һˮ�������c��H+��������ڡ������ڡ���С�ڡ�����
��5��25��ʱ��CH3COOH��CH3COONa�Ļ����Һ������û��ҺpH=6������Һ��c��CH3COO-��-c��Na+��=9.9��10-7mol/L������ȷ��ֵ��
���� ��1���������Խ������ĵ���ƽ�ⳣ��ԽС�����������ˮ��̶�Խ����ͬŨ�ȵ�������Һ���������ˮ��̶�Խ����������ҺpHԽ��
��2����������ˮ�д������Ũ�ȣ�����ˮ�м���������ܺ�ϡ���ᷴӦ�����ܺʹ����ᷴӦ�����ƴ�������룻
��3����ˮϡ�ʹ���ٽ�������룬�������������̶�С����Һ�������̶ȣ�������Һ��c��H+����С���¶Ȳ��䣬ˮ�����ӻ��������䣻
��4����ˮϡ�ʹٽ�������룬pH��ͬ�IJ�ͬ��ϡ����ͬ�ı�����pH�仯���������ǿ���仯С��������������������ˮ���룬���������ӻ��������������Ũ��Խ��������ˮ����̶�Խ��
��5��25��ʱ��CH3COOH��CH3COONa�Ļ����Һ������û��ҺpH=6����c��H+��=10-6 mol/L����Һ��c��OH-��=$\frac{1{0}^{-14}}{1{0}^{-6}}$mol/L=10-8mol/L�����ݵ���غ��c��CH3COO-��-c��Na+��=c��H+��-c��OH-����
��� �⣺��1���������Խ������ĵ���ƽ�ⳣ��ԽС�����������ˮ��̶�Խ����ͬŨ�ȵ�������Һ���������ˮ��̶�Խ����������ҺpHԽ���ݱ�������֪������ǿ��˳����CH3COOH��H2CO3��HClO��HCO3-���������ˮ��̶ȴ�С˳����CO32-��ClO-��HCO3-��CH3COO-������ͬŨ�ȵ�����pH��С˳����A��B��D��C��
�ʴ�Ϊ��A��B��D��C��
��2����������ˮ�д������Ũ�ȣ�����ˮ�м���������ܺ�ϡ���ᷴӦ�����ܺʹ����ᷴӦ�����ƴ�������룬����ǿ��˳����CH3COOH��H2CO3��HClO��HCO3-�����Լ���̼�������ܺ�ϡ���ᷴӦ�����ʹ����ᷴӦ������������������ƴ�������룬��ѡBD��
��3���¶Ȳ��䣬ˮ�����ӻ��������䣬
A����ˮϡ�ʹ���ٽ�������룬�������������̶�С����Һ�������̶ȣ�������Һ��c��H+����С����A��ȷ��
B����ˮϡ�ʹ���ٽ�������룬�������������̶�С����Һ�������̶ȣ�������Һ��c��CH3COO-��С���¶Ȳ������ƽ�ⳣ�����䣬����$\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$=$\frac{c��{H}^{+}����c��C{H}_{3}CO{O}^{-}��}{c��C{H}_{3}COOH����c��C{H}_{3}CO{O}^{-}��}$=$\frac{{K}_{a}}{c��C{H}_{3}CO{O}^{-}��}$����B����
C���¶Ȳ���ˮ�����ӻ��������䣬����c��H+����c��OH-�����䣬��C����
D����ˮϡ�ʹ���ٽ�������룬�������������̶�С����Һ�������̶ȣ�������Һ��c��H+����С�����ӻ��������䣬��c��OH-����������$\frac{c��O{H}^{-}��}{c��{H}^{+}��}$����D����
��ѡA��
��4����ˮϡ�ʹٽ�������룬pH��ͬ�IJ�ͬ��ϡ����ͬ�ı�����pH�仯���������ǿ���仯С��������������������ˮ���룬���������ӻ��������������Ũ��Խ��������ˮ����̶�Խ��
����ͼ֪��pH��ͬ�Ĵ����HXϡ����ͬ�ı�����HX��pH�仯����HX�����Դ��ڴ��ᣬ����HX�ĵ���ƽ�ⳣ�����ڳ�����ϡ�ͺ������������Ũ�ȴ���HX�����Դ�������ˮ����̶ȴ���HX����HX��Һ��ˮ���������c��H+�����ڴ�����Һˮ�������c��H+����
�ʴ�Ϊ�����ڣ����ڣ�
��5��25��ʱ��CH3COOH��CH3COONa�Ļ����Һ������û��ҺpH=6����c��H+��=10-6 mol/L����Һ��c��OH-��=$\frac{1{0}^{-14}}{1{0}^{-6}}$mol/L=10-8mol/L�����ݵ���غ��c��CH3COO-��-c��Na+��=c��H+��-c��OH-��=10-6 mol/L-10-8mol/L=9.9��10-7mol/L��
�ʴ�Ϊ��9.9��10-7mol/L��
���� ���⿼��������ʵĵ��롢����Ũ�ȴ�С�Ƚϵ�֪ʶ�㣬Ϊ��Ƶ���㣬��ȷ����ĵ���ƽ�ⳣ�����������ǿ�����������ˮ��̶ȹ�ϵ�ǽⱾ��ؼ���ע�⣺���е�ƽ�ⳣ����ֻ���¶��йأ�����ҺŨ�ȼ�������أ�Ϊ�״��㣮

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ��ϩ��ʯ���ѽ��IJ��� | |
| B�� | ��������Ҫ������ú�ĸ�����ú���� | |
| C�� | ���͡�ú�͡�������Ҫ������ʯ�͵ij�ѹ���� | |
| D�� | �ѻ����Ͳ���ʹ��ˮ��ɫ |
| A�� | C2H4 | B�� | C4H8 | C�� | C3H8 | D�� | SiO2 |
| A�� | 0.4 mol O2 | B�� | 16g CH4 | ||
| C�� | 3.01��1023��H2SO4 | D�� | ��״����44.8L CO2 |
| A�� | �����£�pH=13��NaOH��Һ�к���OH-����ĿΪ0.1NA | |
| B�� | 25��ʱ��1L pH=13��Ba��OH��2��Һ�к�OH-��ĿΪ0.2NA | |
| C�� | 1L 1mol•L-1��Na2CO3��Һ�к���CO32-����ĿΪNA | |
| D�� | 18g H2O�к�1mol��ԭ�� |
| ������ | Na+ H+ Ba2+ |
| ������ | OH- CO${\;}_{3}^{2-}$ SO${\;}_{4}^{2-}$ |
��A��Һ��B��Һ��Ӧ������ɫ����X������X���Ժ�C��Һ��Ӧ���ɳ���E������E����B��Һ��Ӧ��
��B��Һ��C��Һ��Ӧ���ɰ�ɫ����D������D������ϡ���ᣮ
���������ʵ��������գ�
��1��д������B��C�Ļ�ѧʽ��BH2SO4 CBa��OH��2
��2��д�����з�Ӧ�����ӷ���ʽ��
��A��Һ��C��Һ��Ӧ��Ba2++CO32-=BaCO3����
��B��Һ��̼��������Һ��Ӧ��H++HCO3-=H2O+CO2����
�۽�������C��Һ����������̼�������Һ��Ӧ��Ba2++2OH-+2HCO3-=2H2O+CO32-+BaCO3����
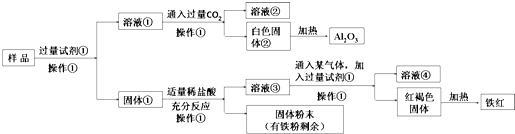
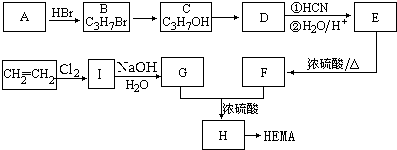
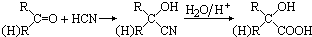
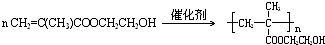 ��
��