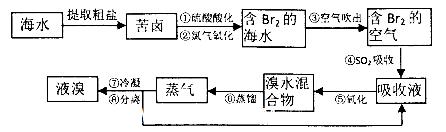
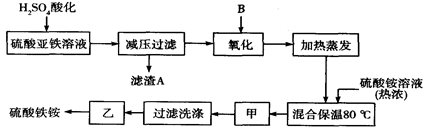
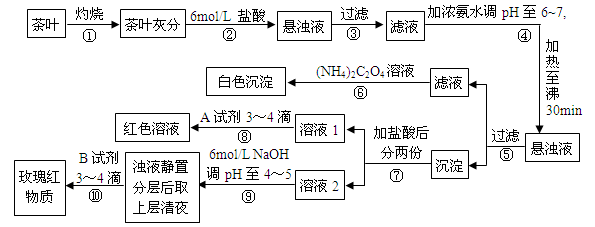
��Ŀ����
��14�֣���ҵ��SnSO4��һ����Ҫ�������Σ��㷺Ӧ���ڶ�����ҵ�����Ʊ�·�����£�

��ʾ������֪�����������£�����ˮ��Һ����Sn2+��Sn4+������Ҫ������ʽ��
����֪Ksp[Sn(OH)2] ��1.0��10-26
�ش��������⣺
��1��SnCl2�����������ˮֱ���ܽ��ԭ����__________������Sn�۵�������_________��
��2����ӦI���ɵij���ΪSnO��д���÷�Ӧ�����ӷ���ʽ___________________________��
��3����������Ѿ���Ưϴ���ɾ��ķ���__________________________________________��
��4����Ӧ�����������֮һ�ǿ�����Һ��pH������Һ��c(Sn2+)��1.0mol?L-1����Ӧ������ҺpH_____��
��5�����������£�SnSO4����������˫��ˮȥ��������д��������Ӧ�����ӷ���ʽ____________��

��ʾ������֪�����������£�����ˮ��Һ����Sn2+��Sn4+������Ҫ������ʽ��
����֪Ksp[Sn(OH)2] ��1.0��10-26
�ش��������⣺
��1��SnCl2�����������ˮֱ���ܽ��ԭ����__________������Sn�۵�������_________��
��2����ӦI���ɵij���ΪSnO��д���÷�Ӧ�����ӷ���ʽ___________________________��
��3����������Ѿ���Ưϴ���ɾ��ķ���__________________________________________��
��4����Ӧ�����������֮һ�ǿ�����Һ��pH������Һ��c(Sn2+)��1.0mol?L-1����Ӧ������ҺpH_____��
��5�����������£�SnSO4����������˫��ˮȥ��������д��������Ӧ�����ӷ���ʽ____________��
��1������Sn2+ˮ�� ��2�֣� ��ֹSn2+��������2�֣���2��Sn2+ + CO32-��SnO�� + CO2����3�֣�
��3��ȡ���һ��ϴ��Һ�������м���AgNO3��Һ������������˵��ϴ�Ӹɾ�����2�֣�
��4��С��1�� ��2�֣� ��5��Sn2+ + H2O2 + 2H+��Sn4+ + 2H2O ��3�֣�
��3��ȡ���һ��ϴ��Һ�������м���AgNO3��Һ������������˵��ϴ�Ӹɾ�����2�֣�
��4��С��1�� ��2�֣� ��5��Sn2+ + H2O2 + 2H+��Sn4+ + 2H2O ��3�֣�
�����������1��SnCl2��ˮ�����ɼ�ʽ�Ȼ�����������ƽ��Sn Cl2+H2O
 Sn��OH��Cl+HCl���������ᣬʹ��ƽ�������ƶ�������Sn2+ˮ�⣻Sn2+�ױ�����������Sn�۳�������ҺpH�⣬����ֹSn2+��������
Sn��OH��Cl+HCl���������ᣬʹ��ƽ�������ƶ�������Sn2+ˮ�⣻Sn2+�ױ�����������Sn�۳�������ҺpH�⣬����ֹSn2+����������2����Ӧ��õ�������SnO��SnԪ�ػ��ϼ�û�б仯�����ڷ�������ԭ��Ӧ��ͬʱ�������壬������Ϊ������̼�����ӷ���ʽΪ��Sn2++CO32-�TSnO��+CO2����
��3�����������������������ӣ���˼�������Ѿ���Ưϴ���ɾ��ķ����ǣ�ȡ���һ��ϴ��Һ�������м���AgNO3��Һ������������˵��ϴ�Ӹɾ���
��4������ Ksp[Sn��OH��2]��1.0��10-26��c��OH-��2��c��Sn2+������c��Sn2+����1.0mol?L-1���˿ɵ�c��OH-����10-13mol/L��c��H+����0.1mol/L����pHС��1��Sn2+��ȫ������
��5�����������£�SnSO4����������˫��ˮȥ������˫��ˮ��ǿ�����ԣ�Sn2+�ױ�����ΪSn4+����������ԭΪˮ�����ӷ���ʽΪ��Sn2++H2O2+2H+�TSn4++2H2O��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 Fe(SCN)3
Fe(SCN)3