��Ŀ����
8�� �ܶ�������ǽ������仯�����ڿ�ѧ�о���ҵ�����ж�����Ҫ��;���ش������й����⣺
�ܶ�������ǽ������仯�����ڿ�ѧ�о���ҵ�����ж�����Ҫ��;���ش������й����⣺��1����̬Crԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d54s1��������Ԫ���л�̬ԭ��δ�ɶԵ�����ΪCr��һ���ҵ縺������Ԫ����N��
��2�������е�ԭ�ӵ��ӻ���ʽΪsp2�������������λ����1������������ص���Է��������ӽ���������������Ϊ�ӷ���Һ�壬����Ϊ���壬�����ԭ�����ط��Ӽ���������ʹ���۷е����ߣ�����������ڴ��������ʹ���۷е㽵�ͣ�
��3������������CO�����������ȣ�������ɫ�ӷ���Һ��Ni��CO��n��Ni��CO��n�����廥Ϊ�ȵ���������ӵĻ�ѧʽΪCN-��C22-��д��һ�����ɣ���
��4��ͭ�ǵ�����������Ҫ�Ĺ���Ԫ��֮һ���䵥�ʼ��������й㷺��;����֪CuH����ṹ��Ԫ��ͼ��ʾ��Hԭ������Խ��ߴ������û�������ܶ�Ϊ�� g•cm-3�������ӵ�������ֵΪNA����þ�����Cuԭ����Hԭ��֮�����̾���Ϊ$\frac{\sqrt{3}}{4}\root{3}{\frac{260}{��•{N}_{A}}}$cm���ú��Ѻ�NA��ʽ�ӱ�ʾ����
���� ��1��Cr��24��Ԫ�أ���ԭ�Ӻ�����24�����ӣ����ݹ���ԭ��д����̬Crԭ�Ӻ�������Ų�ʽ��Crԭ����6��δ�ɶԵ��ӣ�������Ԫ���л�̬ԭ��δ�ɶԵ�����ΪCr��һ���Ԫ����N��P��ͬ���壬Ԫ�صĵ縺������ԭ���������������С��
��2�����ݵ��ļ۲���Ӷ����жϣ�N�������5�����ӣ���һ�����γ�˫����ȥ2�����ӣ�����һ�����γɵ�����ȥ1�����ӣ���ʣ���������ӣ��ṩ������������Ϊ���õ��Ӷԣ�ʹ�������ﵽ8�����ȶ��ṹ���Ӷ��γ���λ�������ط��Ӽ���������ʹ���۷е����ߣ�
��3��Ni��CO��4��������CO��CO�ĵȵ�����������ԭ�Ӹ�������Ҽ۵�������ȵķ��ӻ����ӣ�
��4�����þ�̯������þ����к��е�ͭԭ�Ӻ���ԭ�Ӹ������ٸ����������ܶȺ����֮��Ĺ�ϵʽ���㣬�辧���ı߳�Ϊa��a=$\root{3}{\frac{4��65}{��{N}_{A}}}$���ٸ��ݾ�����Cuԭ����Hԭ��֮�����̾���Ϊ��Խ��ߵ�$\frac{1}{4}$���ݴ˴��⣮
��� �⣺��1��Cr��24��Ԫ�أ���ԭ�Ӻ�����24�����ӣ������������ԭ����д�������Ų�ʽΪ1s22s22p63s23p63d54s1��Crԭ����6��δ�ɶԵ��ӣ�������Ԫ���л�̬ԭ��δ�ɶԵ�����ΪCr��һ���Ԫ����N��P��ͬ���壬Ԫ�صĵ縺������ԭ���������������С����縺��N����P��
�ʴ�Ϊ��1s22s22p63s23p63d54s1��N��
��2�������е�ԭ�Ӻ��������ɼ����Ӷԣ�û�йµ��Ӷԣ���Nԭ�ӵļ۲���Ӷ���Ϊ3������sp2��N�������5�����ӣ���һ�����γ�˫����ȥ2�����ӣ�����һ�����γɵ�����ȥ1�����ӣ���ʣ���������ӣ��ṩ������������Ϊ���õ��Ӷԣ�ʹ�������ﵽ8�����ȶ��ṹ���Ӷ��γ���λ�����������к���һ����λ�������ط��Ӽ���������ʹ���۷е����ߣ�����������ڴ��������ʹ���۷е㽵�ͣ��������ص��۷е�������
�ʴ�Ϊ��sp2��1�����ط��Ӽ���������ʹ���۷е����ߣ�����������ڴ��������ʹ���۷е㽵�ͣ�
��3��CO�����к���2��ԭ�ӣ���۵��Ӹ�����10��������Ni��CO��4�����廥Ϊ�ȵ��ӵ�������CN-��C22-��
�ʴ�Ϊ��CN-��C22-��
��4���þ����к���4��Hԭ�ӣ�ͭԭ�Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4�����Ըþ����к���4��ͭԭ��4����ԭ�ӣ���þ����ı߳�Ϊa����a=$\root{3}{\frac{4��65}{��{N}_{A}}}$���ٸ��ݾ�����Cuԭ����Hԭ��֮�����̾���Ϊ��Խ��ߵ�$\frac{1}{4}$������Խ���Ϊ$\sqrt{3}$a������Cuԭ����Hԭ��֮�����̾���Ϊ$\frac{\sqrt{3}}{4}$��$\root{3}{\frac{4��65}{��{N}_{A}}}$=$\frac{\sqrt{3}}{4}\root{3}{\frac{260}{��•{N}_{A}}}$cm��
�ʴ�Ϊ��$\frac{\sqrt{3}}{4}\root{3}{\frac{260}{��•{N}_{A}}}$��
���� ���⿼���Ϊ�ۺϣ��漰�����ļ��㡢�۵����Ų�ʽ����д���ȵ������֪ʶ�㣬�����ļ�����ѧϰ�ѵ㣬Ӧ�������գ���Ŀ�ѶȽϴ�

 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�| ʵ����� | ʵ������ | ���ͻ���� | |
| A | ���ȣ�170�棩�Ҵ���Ũ�������������������ֱ��ͨ�����Ը��������Һ | ���Ը��������Һ�Ϻ�ɫ��dz | ���ܲ�����SO2���� |
| B | ȡ����KI��Һ���ȵμ���ˮ���ټ����������������� | ��Һ��Ϊ���㣬�²����Ϻ�ɫ | KI����ˮ����������Ӧ |
| C | ������SO2ͨ�뱽������Һ�У��� | ��Һ�г��ֻ��� | ��Ϊ�����˷�Ӧ�� C6H5O-+SO2+H2O�TC6H5OH+HSO3- |
| D | ��Ũ��ˮ���뵽ʢ��Cu2O���Թ��� | ��Һ����ɫ������һ��ʱ�������ɫ | Cu+��������������Cu2+��ʹ��Һ����ɫ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | Beԭ�ӵĽṹʾ��ͼ��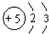 | |
| B�� | ��������ķ���ʽ��SiO2 | |
| C�� | ������Ϊ53��������Ϊ78�ĵ�ԭ�ӿɱ�ʾΪ��53131I | |
| D�� | ��ϩ�Ľṹʽ��CH2=CH2 |
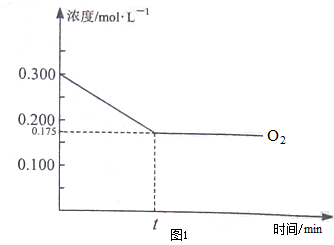

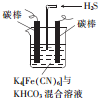 �����仯�������ճ�������Ӧ�ù㷺��
�����仯�������ճ�������Ӧ�ù㷺��