��Ŀ����
15��A��B��C��D��E����5��Ԫ�أ���1��AԪ�ػ�̬ԭ�ӵ��������3��δ�ɶԵ��ӣ��������2�����ӣ�A����̬�⻯�ﻯѧʽΪNH3
��2��BԪ�صĸ�һ�����Ӻ�CԪ�ص���һ�����ӵĵ��Ӳ�ṹ�������ͬ��B������������ˮ����Ļ�ѧʽΪHClO4
��3��DԪ�ص����������ӵ�3d�Dz�Ϊ�������D��Ԫ�ط���ΪFe
��4��EԪ�ػ�̬ԭ�ӵ�M��ȫ������N��û�гɶԵ��ӣ�ֻ��һ��δ�ɶԵ��ӣ����̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d104s1��
���� ��1��AԪ�ػ�̬ԭ�ӵ��������3��δ�ɶԵ��ӣ���������Ų�ӦΪns2np3��
��2��BԪ�صĸ�һ�����Ӻ�CԪ�ص���һ�����ӵĵ��Ӳ�ṹ�������ͬ�����Ӻ��ⶼ��18�����ӣ��ݴ˼��������������жϣ�
��3��DԪ�ص����������ӵ�3d���Ϊ�������3d���������Ϊ5��ӦΪFeԪ�أ�
��4��EԪ�ػ�̬ԭ�ӵ�M��ȫ������N��û�гɶԵ��ӣ�ֻ��һ��δ�ɶԵ��ӣ�ӦΪCu��ԭ������Ϊ29��
��� �⣺��1��AԪ�ػ�̬ԭ�ӵĺ�������Ų�ӦΪns2np3���������2�����ӣ�������Ų�ʽΪ��1s22s22p3��ӦΪNԪ�أ�����̬�⻯�ﻯѧʽΪNH3��
�ʴ�Ϊ��NH3��
��2��BԪ�صĸ�һ�����Ӻ�CԪ�ص���һ�����ӵĵ��Ӳ�ṹ�������ͬ�����Ӻ��ⶼ��18�����ӣ�BԪ��������Ϊ18-1=17��BΪ��Ԫ�أ�Ԫ�ط���ΪCl��������������ˮ����Ļ�ѧʽΪHClO4��
�ʴ�Ϊ��HClO4��
��3��DԪ�ص����������ӵ�3d���Ϊ�������3d���������Ϊ5�����̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d64s2��ӦΪFeԪ�أ�
�ʴ�Ϊ��Fe��
��4��EԪ�ػ�̬ԭ�ӵ�M��ȫ������N��û�гɶԵ��ӣ�ֻ��һ��δ�ɶԵ��ӣ���̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d104s1��ӦΪCuԪ�أ�
�ʴ�Ϊ��1s22s22p63s23p63d104s1��
���� ���⿼��Ԫ���ƶϡ���������Ų��ȣ���Ŀ�ѶȲ���ע������ԭ�Ӻ�����ӵ��Ų����ɣ��Դ��ƶ�Ԫ�أ�ע�����֪ʶ���������գ�

 ������ϵ�д�
������ϵ�д� �żӾ���ϵ�д�
�żӾ���ϵ�д�| A�� | ԭ�Ӽ������ӵĺ�����Ӳ������ڸ�Ԫ�����ڵ������� | |
| B�� | Ԫ�����ڱ��д�IIIB�嵽IIB�� 10�����е�Ԫ�ض��ǽ���Ԫ�� | |
| C�� | �ڢ�A��Ԫ�صĽ����Աȵڢ�A��Ԫ�صĽ�����ǿ | |
| D�� | �����ڱ������Ԫ�����ڵ�����������ԭ�Ӻ�������� |
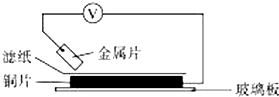 ���ྻ�Ľ���ƬA��B��C��D�ֱ�����ڽ���ij������Һ����ֽ���沢ѹ������ͼ��ʾ������ÿ��ʵ��ʱ����¼��ѹָ����ƶ�����͵�ѹ���Ķ������±�����֪���������缫�Ľ�����������������Խ��ѹ���Ķ���Խ�������ݼ�¼�����жϣ������й�˵����ȷ���ǣ�������
���ྻ�Ľ���ƬA��B��C��D�ֱ�����ڽ���ij������Һ����ֽ���沢ѹ������ͼ��ʾ������ÿ��ʵ��ʱ����¼��ѹָ����ƶ�����͵�ѹ���Ķ������±�����֪���������缫�Ľ�����������������Խ��ѹ���Ķ���Խ�������ݼ�¼�����жϣ������й�˵����ȷ���ǣ�������| ���� | ������������ | ��ѹ/V |
| A | A��Cu | +0.78 |
| B | Cu��B | +0.15 |
| C | C��Cu | +1.35 |
| D | D��Cu | +0.30 |
| A�� | �����ֽ�����C�Ļ�ԭ������ | |
| B�� | ����B�ܴ�����ͭ��Һ���û���ͭ | |
| C�� | AD���γ�ԭ���ʱAΪ���� | |
| D�� | AB�γɺϽ�ʱ�����úϽ�¶���ڿ����У�A�ȱ���ʴ |
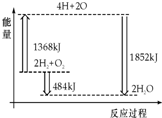
| A�� | H2��g����O2��g����Ӧ����H2O��g �����������ȷ�Ӧ | |
| B�� | 2 mol H2��1 mol O2ת��Ϊ4 mol H��2 mol Oԭ�ӵĹ����зų����� | |
| C�� | 2 mol H2��g����1 mol O2��g����Ӧ����2 mol H2O�� g�������ų�484 kJ���� | |
| D�� | 4 mol H��2 mol O����2 mol H2O��g�������ų�484 kJ���� |
| ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ����A | 0 |
| �� | �� | �� | ||||||
| �� | �� | �� | �� | �� | �� | �� | ||
| �� | �� | �� |
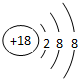 ��
����2���ڶ������У�Ԫ�ص�����������Ӧ��ˮ������������ǿ����HClO4��������ǿ����KOH�������Ե�����������Al��OH��3��
��3���ڢڢߢ�Ԫ���У�ԭ�Ӱ뾶������Br��ԭ�Ӱ뾶��С����F��
��4���ڢ����ĵ����У���ѧ���ʽϻ��õ���K��д������ˮ��Ӧ�Ļ�ѧ����ʽ��2K+2H2O=2KOH+H2����
| A�� | �����£�1L 1mol•L-1��NH4NO3��Һ�е�ԭ����Ϊ0.2NA | |
| B�� | 1mol�ǻ��е�����Ϊ10NA | |
| C�� | �ڷ�Ӧ�У�ÿ����3mol I2ת�Ƶĵ�����Ϊ6NA | |
| D�� | ���³�ѹ�£�22.4L��ϩ��C-H����Ϊ4NA |
| A�� | ������Һ�д���������ӣ���֤��������һ������ | |
| B�� | ��ȩ����ȩ����ȩ�������ܺ�������Һ������������ͭ��Ӧ | |
| C�� | ��ȥ��ҵ�Ҵ�������ˮ��������ʯ�ң������� | |
| D�� | ��֬�����ۡ������ʡ���ά��������Ȼ�߷��ӻ����� |

�й���Ϣ���£�
�ٷ�Ӧԭ����TiO2��s��+CCl4��g��$\frac{\underline{\;\;��\;\;}}{\;}$ TiCl4��g��+CO2��g��
�ڷ�Ӧ��������ˮ�����Ҽ���
���й������������±���
| ���� | �۵�/�� | �е�/�� | ���� |
| CCl4 | -23 | 76 | ��TiCl4���� |
| TiCl4 | -25 | 136 | ����ʪ������������ |
��1������A�����������θ���ܣ�����װ���Լ��������ǣ�����ţ�B��
A����ʯ�� B����ˮ����ͭ C�������� D���ռ�
��2������ʵ�������ͨN2��Ŀ�����ų�װ���еĿ�������֤��Ӧ����ˮ���������½��У�
��3��װ��E�е��Լ���Ũ�����ȤС��ļ�ͬѧ��Ϊʵ����Eװ�ò��ܻ���Aװ�ã�������Aװ�ò������տ����е����������ܱ�֤��Ӧ�����������½���
��4��ʵ�鿪ʼǰ�IJ�����������װ���������װ�õ������ԡ���װҩƷ��ͨN2һ��ʱ����ȼ�ƾ��ƣ�
��5��������D�е�Һ̬���������ò���������������
��6��TiCl4������TiO2�ͽ�̿�������ڼ����·�Ӧ�Ƶã�ͬʱ��CO��������Ӧ�Ļ�ѧ����ʽΪTiO2+2Cl2+2C $\frac{\underline{\;\;��\;\;}}{\;}$TiCl4+2CO����Ӧ����������ͨ����ⱥ��ʳ��ˮ�Ƶã��ֵ��1L����ʳ��ˮ����ת�Ƶĵ�����Ϊ0.1NAʱ������Һ��pHֵΪ13��
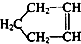 �ɼ�дΪ
�ɼ�дΪ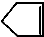 ������Ƭϩ�ķ��ӽṹ�ɱ�ʾΪ��
������Ƭϩ�ķ��ӽṹ�ɱ�ʾΪ��