题目内容
二氧化锰可用作干电池去极剂,合成工业的催化剂和氧化剂,玻璃工业和搪瓷工业的着色剂、消色剂、脱铁剂等。
(1)二氧化锰在酸性介质中是一种强氧化剂,请用化学方程式证明:______________________。
(2)锌—锰碱性电池具有容量大、放电电流大的特点,因而得到广泛应用。电池的总反应式为Zn(s)+2MnO2(s)+H2O(l)=Zn(OH)2(s)+Mn2O3(s)。
①电池工作时,MnO2发生________反应。
②电池的正极反应式为________。
(3)工业上以软锰矿为原料,利用硫酸亚铁制备高纯二氧化锰的流程如下:

已知:软锰矿的主要成分为MnO2,还含Si(16.27%)、Fe(5.86%)、Al(3.42%)、Zn(2.68%)和Cu(0.86%)等元素的化合物,部分阳离子以氢氧化物或硫化物的形式完全沉淀时溶液的pH见下表。
回答下列问题:
①硫酸亚铁在酸性条件下将MnO2还原为MnSO4,酸浸时发生的主要反应的化学方程式为________________________。
②试剂X为________。
③滤渣A的主要成分为________。
④加入MnS的目的主要是除去溶液中的________。
(1)二氧化锰在酸性介质中是一种强氧化剂,请用化学方程式证明:______________________。
(2)锌—锰碱性电池具有容量大、放电电流大的特点,因而得到广泛应用。电池的总反应式为Zn(s)+2MnO2(s)+H2O(l)=Zn(OH)2(s)+Mn2O3(s)。
①电池工作时,MnO2发生________反应。
②电池的正极反应式为________。
(3)工业上以软锰矿为原料,利用硫酸亚铁制备高纯二氧化锰的流程如下:

已知:软锰矿的主要成分为MnO2,还含Si(16.27%)、Fe(5.86%)、Al(3.42%)、Zn(2.68%)和Cu(0.86%)等元素的化合物,部分阳离子以氢氧化物或硫化物的形式完全沉淀时溶液的pH见下表。
| 沉淀物 | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Mn(OH)2 | Cu(OH)2 |
| pH | 5.2 | 3.2 | 9.7 | 10.4 | 6.7 |
| 沉淀物 | Zn(OH)2 | CuS | ZnS | MnS | FeS |
| pH | 8.0 | -0.42 | 2.5 | 7 | 7 |
回答下列问题:
①硫酸亚铁在酸性条件下将MnO2还原为MnSO4,酸浸时发生的主要反应的化学方程式为________________________。
②试剂X为________。
③滤渣A的主要成分为________。
④加入MnS的目的主要是除去溶液中的________。
(1)MnO2+4HCl(浓) MnCl2+Cl2↑+2H2O
MnCl2+Cl2↑+2H2O
(2)①还原 ②2MnO2+2e-+H2O=Mn2O3+2OH-
(3)①MnO2+2FeSO4+2H2SO4=MnSO4+
Fe2(SO4)3+2H2O
②氨水 ③Fe(OH)3和Al(OH)3 ④Cu2+、Zn2+
 MnCl2+Cl2↑+2H2O
MnCl2+Cl2↑+2H2O(2)①还原 ②2MnO2+2e-+H2O=Mn2O3+2OH-
(3)①MnO2+2FeSO4+2H2SO4=MnSO4+
Fe2(SO4)3+2H2O
②氨水 ③Fe(OH)3和Al(OH)3 ④Cu2+、Zn2+
(1)二氧化锰在酸性介质中是一种强氧化剂,可联想到实验室制取氯气。(2)MnO2在正极得电子,发生还原反应,电极反应式为2MnO2+2e-+H2O=Mn2O3+2OH-。(3)①根据FeSO4在反应条件下将MnO2还原为MnSO4,Fe2+被氧化为Fe3+,可以写出反应方程式:2FeSO4+MnO2+2H2SO4=MnSO4+Fe2(SO4)3+2H2O。②调节pH至5.4,是为了使Fe3+、Al3+沉淀,所选试剂为氨水。③滤渣A的主要成分为Fe(OH)3和Al(OH)3。④根据流程图可知加入MnS是为了生成CuS、ZnS而除去Cu2+、Zn2+。

练习册系列答案
相关题目
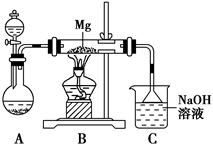
 2MgSO3+S;丙同学的推测是:3Mg+SO2
2MgSO3+S;丙同学的推测是:3Mg+SO2