��Ŀ����
�����й�ʵ��ԭ������������������۲���ȷ����(����)
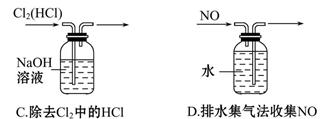
| A����ȥ����CO2�л��е�����SO2,�ɽ������������ͨ��ʢ������KMnO4��Һ��Ũ�����ϴ��ƿ |
| B����ˮʪ��pH��ֽ����ij��Һ��pH,һ�������ʵ����� |
| C������ƿ����ƿ������ˮϴ����ʹ��,�ζ���������ˮϴ����,��������ʢ��Һ��ϴ���κ�ʹ�� |
| D����ȡa��Na2CO3��NaHCO3������ּ���,����b��,�ɲⶨ�������Na2CO3���������� |
B
SO2�ܱ����Ը��������Һ������ȥ,A����ȷ;����Һ������,��ˮʪ���pH��ֽ����pH�������ʵ�����,B�����;�к͵ζ�ʱ��ƿ������ƿ������ϴ,���ζ���Ҫ����ʢҺ��ϴ2��3�κ�ʹ��,C����ȷ;ֻ��NaHCO3���ȷֽ�����Na2CO3ʹ�������������,�����ò���������������Na2CO3����������,D����ȷ��

��ϰ��ϵ�д�
�����Ŀ
 ����NH4F+ ����H2SiO3��+����CO2��+��������
����NH4F+ ����H2SiO3��+����CO2��+��������