��Ŀ����
������һ���ḻ����Դ���⣬ͨ����ˮ���ۺ����ÿɻ���������ʹ�����ʹ�á�
��1�� ��ˮ���εĿ������ã�
��.��ˮ����Ŀǰ�����Ϊ�������������ѡ��Զ�뽭���뺣�ڣ�������꣬��ϫ��������ƽ̹�տ��ĺ�̲�����������Ϊ��ˮ�ء������غ�_______�ء�
II.Ŀǰ��ҵ�ϲ��ñȽ��Ƚ������ӽ���Ĥ���۷������ȼҵ�������ڵ����������ӽ���Ĥֻ����������ͨ������ֹ�����Ӻ�����ͨ������˵���ȼ������������ӽ���Ĥ������____________________________________________����дһ�㼴�ɣ�
��2�����������ǽ�������չ������һ�ֽϺõĺ�ˮ������������ԭ������ͼ��ʾ����ش��������⣺
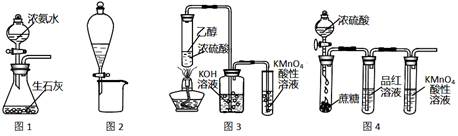
��.��ˮ����ֱ��ͨ�뵽��װ���У�������_____________________________________________��
��. B���ų�����________(���ˮ����Ũˮ��)��
��3���ÿ�±����Na+��K+��Mg2+��Cl-��Br-�����ӣ�����ȡ�壬�������������£�
��.���������е���Һ��BrO3�������������з�Ӧ�����ӷ���ʽΪ�ߣߣߣߣߣߣߣߣߣߣߣߣ�
_________________________________________��
��.ͨ�����Ȼ��ѻ�ú�Br2����Һ��Ϊ�λ��辭�����������ա��ữ�����»�ú�Br2����Һ��_____________________________________________________________________��
��.����������ͨ��ˮ�������ȣ������¶���900C���ҽ��������ԭ����___________________________________________________________________________��
��1�� ��ˮ���εĿ������ã�
��.��ˮ����Ŀǰ�����Ϊ�������������ѡ��Զ�뽭���뺣�ڣ�������꣬��ϫ��������ƽ̹�տ��ĺ�̲�����������Ϊ��ˮ�ء������غ�_______�ء�
II.Ŀǰ��ҵ�ϲ��ñȽ��Ƚ������ӽ���Ĥ���۷������ȼҵ�������ڵ����������ӽ���Ĥֻ����������ͨ������ֹ�����Ӻ�����ͨ������˵���ȼ������������ӽ���Ĥ������____________________________________________����дһ�㼴�ɣ�
��2�����������ǽ�������չ������һ�ֽϺõĺ�ˮ������������ԭ������ͼ��ʾ����ش��������⣺

��.��ˮ����ֱ��ͨ�뵽��װ���У�������_____________________________________________��
��. B���ų�����________(���ˮ����Ũˮ��)��
��3���ÿ�±����Na+��K+��Mg2+��Cl-��Br-�����ӣ�����ȡ�壬�������������£�

��.���������е���Һ��BrO3�������������з�Ӧ�����ӷ���ʽΪ�ߣߣߣߣߣߣߣߣߣߣߣߣ�
_________________________________________��
��.ͨ�����Ȼ��ѻ�ú�Br2����Һ��Ϊ�λ��辭�����������ա��ữ�����»�ú�Br2����Һ��_____________________________________________________________________��
��.����������ͨ��ˮ�������ȣ������¶���900C���ҽ��������ԭ����___________________________________________________________________________��
��1�� �� �ᾧ �� ��ֹH2��Cl2������Ӧ����������ը����ֹCl2�����ɵ�NaOH��Һ��Ӧ��ʹ�ռ��Ʒ������ ������������Ҳ�Ʒ֣�
��2���� ��ˮ�к��϶�Mg2+ ��Ca2+�������ӣ����ʱ�����Mg(OH)2��Ca(OH)2�ȳ����Ӷ����������ӽ���Ĥ �������𰸾��Ʒ֣� ��3�֣� �� ��ˮ
��3����3CO32��+ 3Br2 �� 5Br��+BrO3��+3CO2��
���壬���Br2��Ũ�� �������𰸾��Ʒ֣�
���¶ȹ������Խ�Br2�������������¶ȹ����ֻὫ������ˮ��������������𰸾��Ʒ֣�
��2���� ��ˮ�к��϶�Mg2+ ��Ca2+�������ӣ����ʱ�����Mg(OH)2��Ca(OH)2�ȳ����Ӷ����������ӽ���Ĥ �������𰸾��Ʒ֣� ��3�֣� �� ��ˮ
��3����3CO32��+ 3Br2 �� 5Br��+BrO3��+3CO2��
���壬���Br2��Ũ�� �������𰸾��Ʒ֣�
���¶ȹ������Խ�Br2�������������¶ȹ����ֻὫ������ˮ��������������𰸾��Ʒ֣�
�����������1���� �δ���ˮ���о��������أ��õ�Ũ�Ƚϴ��±ˮ���ٽ�±ˮת�Ƶ��ᾧ���нᾧ�����յô��Σ��������ӽ���Ĥֻ����������ͨ������ֹ�����Ӻ�����ͨ�����Ӷ��������������������������������������Ͳ�����Ӵ���Ӧ��������ը��ͬʱ�����ϲ�������������Ҳ������������Ӧ�����Ƚϸߣ�
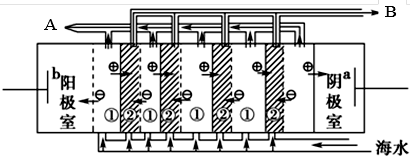
��2���� ��ˮ�и���Ca2+��Mg2+���ӣ�������ˮֱ��ͨ�뵽��װ���У���ˮ�е�Ca2+��Mg2+�����������������ӽ�ϲ���Mg(OH)2��Ca(OH)2�ȳ����Ӷ����������ӽ���Ĥ �� ͼ�Тڴ���ֱ���糡�������£���Һ�е����Ӿ�������Ǩ�ơ���Ĥֻ����������ͨ�����������ӽ�����������Ĥֻ����������ͨ�����������ӽ������������ʹ��ЩС�ҵ�һ���ֱ�ɺ����Ӻ��ٵĵ�ˮ�ң���ˮ��Ϊ��ˮ�����뵭ˮ�����ڵ�С�����ɾۼ��������ӵ�Ũˮ�ң���ˮ��ΪŨˮ������B����ˮΪ��ˮ��
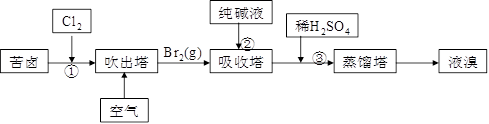
��3������̼���ƣ����巴Ӧ��BrO3�����ɣ���Ӧ�����ӷ���ʽΪ3CO32��+ 3Br2 �� 5Br��+BrO3��+3CO2��
��Ӣٳ�������Һ����ĺ������ߣ����ֱ������Ʒ�ɱ��ߣ�������Ҫ��һ��Ũ���壬������Ũ�ȣ�
�� �¶ȹ���ˮ�������������к���ˮ�֣��¶ȹ����岻����ȫ���������ʵ͡�

��ϰ��ϵ�д�
 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д�
�����Ŀ
 ����NH4F+ ����H2SiO3��+����CO2��+��������
����NH4F+ ����H2SiO3��+����CO2��+��������  ������I
������I
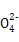 ��N
��N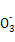 ��Cl-�е�4������,�������ӵ����ʵ�����Ϊ1 mol���������Һ�м��������ϡ����,�����ݲ���,����Һ�������������(������ˮ�ĵ�������ӵ�ˮ��)������˵������ȷ����( )
��Cl-�е�4������,�������ӵ����ʵ�����Ϊ1 mol���������Һ�м��������ϡ����,�����ݲ���,����Һ�������������(������ˮ�ĵ�������ӵ�ˮ��)������˵������ȷ����( )