��Ŀ����
11����ͼΪijƷ�ƽ��ͱ�ǩ��һ���֣�
��1��������̬���ĺ����ǽ�����������Ҫָ�꣬��Щ���������ɴ��е���ҪӪ�����ʵ�����ˮ������ģ�
��2�������嵥�����ڷ��������DZ������ƣ�������ɫ�����ǽ���ɫ
��3��С���е���ҪӪ�������ǵ��ۣ������ƣ�������������ȫˮ��IJ�����C6H12O6���ѧʽ����
��4��������Ӫ�����ġ�ǿ�����������Ѿ����У���Ԫ�������������������������Ԫ�أ������������ı���ÿ100mL ��������Ӫ��������������200mg��Ӫ��������Ԫ�ص���������Ϊ12.5%���ý����е���Ԫ��ֻ��10%�ܱ��������գ����ʹ��16mL �ý��ͣ��ܴӽ��������յ���Ԫ��Ϊ0.4mg�����൱�ڷ��ò�����FeSO4 1.1mg���������һλС������
���� ��1��������ˮ�����ɰ����
��2������������Ӧ�úܹ�Ļ�ѧ�������������Ի���������������������
��3��С���е���ҪӪ�������ǵ��ۣ�����ˮ�����������ǣ�
��4����Ԫ����Ҫ�У������ܡ�ͭ��п�������̡��⡢�����⡢�����������⣬ÿ100mL��������Ӫ������������200mg��������Ԫ����������Ϊ12.5%�������е���ֻ��10%�ܱ��������գ��ݴ˼����ʳ��16mL�ý��ͣ��ܴӽ��������յ���Ԫ�أ���ϻ�������ijԪ�ص�����=�û��������������Ԫ�ص�����������
��� �⣺��1�����������ʣ�������ˮ�����ɰ����ᣬ�ʴ�Ϊ�������ʣ�
��2����������������Ϣ�����ƣ������Խϴ��״�ϸ��Ĥ����ϸ�����ڣ�����ϸ��Ĥ��ͨ�ԣ�����ϸ��Ĥ����������գ��������ƽ���ϸ�����ڵ����ữϸ���ڵļ��������ϸ���ĺ���øϵ�Ļ��ԣ��Ӷ���ʳƷ������Ŀ�ģ��dz��õ�ʳƷ���������ʴ�Ϊ���������ƣ�
��3��С���е���ҪӪ�������ǵ��ۣ���ȫˮ��Ļ�ѧ����ʽ����C6H10O5��n+n H2O$\stackrel{����}{��}$n C6H12O6���ʴ�Ϊ�����ۣ�C6H12O6��
��4������������������Ԫ�أ�ÿ100mL��������Ӫ������������200mg��������Ԫ����������Ϊ12.5%�������е���ֻ��10%�ܱ��������գ���ʳ��16mL�ý��ͣ��ܴӽ��������յ���Ԫ�ص�����Ϊ200mg��$\frac{16mL}{100mL}$��12.5%��10%=0.4mg�����൱�ڷ��ò�����FeSO4������Ϊ0.4mg�£�$\frac{56}{56+32+16��4}$��100%����1.1mg��
�ʴ�Ϊ������0.4��1.1��
���� ���⿼�黯ѧ������漰������ˮ�⡢ʳƷ�������������ơ�С���е���ҪӪ�����ʡ���ǿ�����͵�֪ʶ�����ػ���֪ʶ�Ŀ��飬ƽʱע�⻯ѧ������֪ʶ�Ļ��ۣ���Ŀ�ѶȲ���

 ��У����ϵ�д�
��У����ϵ�д�| A�� | ��100mL��Ͳ��ȡ23.25mLŨ������Һ | |
| B�� | �ö��Ե缫���NaCl��Һ��һ��ʱ����ټ����ᣬ��ʹ��Һ��ԭ��Һ��ȫһ�� | |
| C�� | ��ϴ��ʽ�ζ���ʱӦ�ӵζ����Ͽڼ���3��5mL��Ҫʢװ������Һ����б��ת���ζ��ܣ�ʹҺ����ʪ���ڱڣ��ٴ��Ͽڵ������ظ�2��3�� | |
| D�� | ����֪Ũ�ȵ����Ը��������Һ�ζ�δ֪Ũ�Ȳ����ʵ���в��ü�ָʾ�� |
| A�� | ����Ϊǿ�ᡢ��Ϊ���ᣬ��c���ף���c���ң�=10��1 | |
| B�� | ����Ϊ���ᣬ��Ϊǿ�ᣬ��һ������c���ף���c���ң� | |
| C�� | ����Ϊǿ�ᣬ��Ϊ���ᣬ�������ʵ���Ũ�Ȳ�������� | |
| D�� | ���ס��Ҿ�Ϊ���ᣬ�����ĵ��볣��һ��������� |
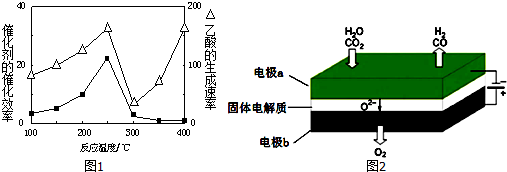
��1��250��ʱ�������Ͻ�Ϊ��������4L������ͨ��6mol CO2��6mol CH4���������·�Ӧ��CO2 ��g��+CH4��g��?2CO��g��+2H2��g����ƽ����ϵ�и��������������±���
| ���� | CH4 | CO2 | CO | H2 |
| ������� | 0.1 | 0.1 | 0.4 | 0.4 |
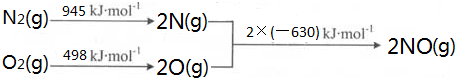
����֪��CH4��g��+2O2��g��=CO2��g��+2H2O��g����H=-890.3kJ•mol-1
CO��g��+H2O ��g��=CO2��g��+H2 ��g����H=+2.8kJ•mol-1
2CO��g��+O2��g��=2CO2��g����H=-566.0kJ•mol-1
��ӦCO2��g��+CH4��g��?2CO��g��+2H2��g�� �ġ�H=+247.3kJ•mol-1
��2���Զ������ѱ��渲��Cu2Al2O4Ϊ���������Խ�CO2��CH4ֱ��ת�������ᣮ
���ڲ�ͬ�¶��´����Ĵ�Ч�������������������ͼ1��ʾ��250��300��ʱ���¶����߶�������������ʽ��͵�ԭ�����¶ȳ���250��ʱ�������Ĵ�Ч�ʽ���
��Ϊ����߸÷�Ӧ��CH4��ת���ʣ����Բ�ȡ�Ĵ�ʩ������Ӧѹǿ������CO2��Ũ��
�۽�Cu2Al2O4�ܽ���ϡ�����е����ӷ���ʽΪ3Cu2Al2O4+32H++2NO3-=6Cu2++6Al3++2NO��+16H2O
��3��Li2O��Na2O��MgO��������CO2�������Ѱ������CO2���������ʣ����н����������ab
a�����ڼ�����������Ѱ��
b�����ڢ�A����A��Ԫ���γɵ���������Ѱ��
c�����ھ���ǿ�����Ե�������Ѱ��
��Li2O����CO2�������ںϳ�Li4SiO4��Li4SiO4�������ա��ͷ�CO2��ԭ���ǣ���500�棬CO2��Li4SiO4�Ӵ�������Li2CO3��ƽ��������700�棬��Ӧ������У��ų�CO2��Li4SiO4������˵����ԭ���Ļ�ѧ����ʽ��CO2+Li4SiO4?Li2CO3+Li2SiO3
��4�����÷�ӦA�ɽ��ͷŵ�CO2ת��Ϊ���й�ҵ���ü�ֵ�IJ�Ʒ����ӦA��CO2+H2O$\frac{\underline{\;���\;}}{����}$CO+H2+O2
���µ�⼼���ܸ�Чʵ�֣�3���з�ӦA������ԭ��ʾ��ͼ��ͼ2��CO2�ڵ缫a�ŵ�ķ�Ӧʽ��CO2+2e-�TCO+O2-��

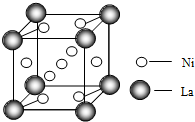 ����28Ni���ڽ����ʻ�������[����Ԫ�غ�һ����̼��CO�����Է����γɵ�һ�������]�������������[�ܿ���Ķ�����ա�������ͷ�������H2���ĺϽ�]��������;�㷺��
����28Ni���ڽ����ʻ�������[����Ԫ�غ�һ����̼��CO�����Է����γɵ�һ�������]�������������[�ܿ���Ķ�����ա�������ͷ�������H2���ĺϽ�]��������;�㷺��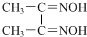 �T
�T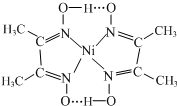 +2H+
+2H+