��Ŀ����
3��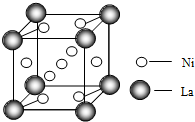 ����28Ni���ڽ����ʻ�������[����Ԫ�غ�һ����̼��CO�����Է����γɵ�һ�������]�������������[�ܿ���Ķ�����ա�������ͷ�������H2���ĺϽ�]��������;�㷺��
����28Ni���ڽ����ʻ�������[����Ԫ�غ�һ����̼��CO�����Է����γɵ�һ�������]�������������[�ܿ���Ķ�����ա�������ͷ�������H2���ĺϽ�]��������;�㷺����1����ԭ�ӻ�̬��������Ų�ʽΪ1s22s22p63s23p63d84s2��[Ar]3d84s2��
��2��Ni��CO��4�����Ļ��ϼ�Ϊ0��д����CO��Ϊ�ȵ���������Է��ӡ���һ����λ����������ӡ���һ����λ����������Ӹ�һ����N2��NO+��CN-��
��3��һ�ִ���Ͻ��������磨La����ɣ��侧��ṹ��ͼ����þ���Ļ�ѧʽΪLaNi5����Ni5La����
��4�����з�Ӧ����������Ni2+����д����һ����Ļ�ѧʽ��
Ni2+
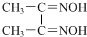 �T
�T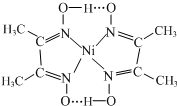 +2H+
+2H+��Ni2+��λ��Nԭ����4������������д��ڵĻ�ѧ����AC������ţ���
A�����ۼ� B�����Ӽ� C����λ�� D�������� E�������
���� ��1�����ݹ���ԭ��д����̬Niԭ�Ӻ�������Ų�ʽ��
��2������Ԫ�ػ��ϼ۴����͵���������𣻸��ݵȵ�������ԭ������ͬ���۵�������ͬ������
��3�����ݾ�̯�����㾧�����磨La��������Ni����ԭ����Ŀ���ݴ�ȷ����ѧʽ��
��4�����������غ�������غ�����ƽ����ԭ���пչ������ԭ���йµ��Ӷԣ���˶����γ���λ�����ǽ�����ǽ���ԭ��֮���γɹ��ۼ������������N-O��Oԭ�Ӻ�-OH����ԭ���γ������
��� �⣺��1��Ni��ԭ������Ϊ28�����ݹ���ԭ����д�������Ų�ʽΪ��1s22s22p63s23p63d84s2��[Ar]3d84s2��
�ʴ�Ϊ��1s22s22p63s23p63d84s2��[Ar]3d84s2��
��2��COΪ���ӣ����ϼ�Ϊ0���������Ļ��ϼ�Ϊ0��CO����2��ԭ��14�����ӣ�����CO��Ϊ�ȵ���������Է��ӡ���һ����λ����������ӡ���һ����λ����������ӷֱ�Ϊ��N2��NO+��CN-��
�ʴ�Ϊ��0��N2��NO+��CN-��
��3���þ�����Laԭ�Ӹ���=8��$\frac{1}{8}$=1��Niԭ�Ӹ���=1+8��$\frac{1}{2}$=5����ѧʽΪLaNi5����Ni5La�����ʴ�Ϊ��LaNi5����Ni5La����
��4���������غ������غ��֪��Ni2+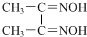 �T
�T +2H+��
+2H+��
Ni���пչ����Nԭ�Ӻ��йµ��Ӷԣ�������λ����Nԭ��ָ��Niԭ�ӣ���4����λ�������������N-O��Oԭ�Ӻ�-OH����ԭ���γ��������������������λ�������Ϊ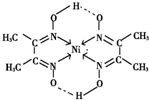 ������֮����й��ۼ�������������ڻ�ѧ����
������֮����й��ۼ�������������ڻ�ѧ����
�ʴ�Ϊ��H+��4��AC��
���� ���⿼���˼۵��ӵ��Ų�ʽ����λ����ԭ���ӻ���ʽ�жϵ�֪ʶ�㣬��Ŀ�Ѷ��еȣ��ѵ�����λ����ʾ������

 ��ѧ�����ϵ�д�
��ѧ�����ϵ�д� �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д����� ��� | �� | �� | �� |
| A | Cl2 | MgBr2 | NaOH |
| B | SO2 | Ca��OH��2 | NaHCO3 |
| C | SiO2 | NaOH | HF |
| D | NH3 | O2 | HNO3 |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | v��A��=0.5 mol/��L•min�� | B�� | v��B��=0.02mol/��L•s�� | ||
| C�� | v��C��=0.8 mol/��L•min�� | D�� | v��D��=0.01mol/��L•s�� |
| A�� | Xԭ�ӵ������������ͺ˵�����϶�Ϊ���� | |
| B�� | X�����γɻ�ѧʽΪKXO3�ĺ�������� | |
| C�� | X���γɻ�ѧʽΪX��OH��3�ļ� | |
| D�� | X����ijЩ����Ԫ���γ����ӻ����� |
| A�� | SO42-��NO3- | B�� | NO3-��Cl- | C�� | SO32-��NO3- | D�� | Cl-��NO3-��Na+ |
| A�� | 4g ���� | B�� | 1.25mol O2 | ||
| C�� | 22.4�� Cl2����״���� | D�� | 6.02��1023��SO2���� |
| A�� | 10mL H2O | B�� | 0.8mol NaOH | C�� | 54 g Al | D�� | 1 g H3PO4 |

 ��1�����淴ӦN2��g��+3H2��g��?2NH3��g����һ�����ȷ�Ӧ���м���������ȫ��ͬ����������������м���1molN2��3molH2��һ�������£��ﵽƽ��ʱ�ų�������ΪQ1����ͬ�����£����������м���2molNH3���ﵽƽ��ʱ�����յ�����ΪQ2����֪Q2=4Q1�����������H2��ת����Ϊ20%��
��1�����淴ӦN2��g��+3H2��g��?2NH3��g����һ�����ȷ�Ӧ���м���������ȫ��ͬ����������������м���1molN2��3molH2��һ�������£��ﵽƽ��ʱ�ų�������ΪQ1����ͬ�����£����������м���2molNH3���ﵽƽ��ʱ�����յ�����ΪQ2����֪Q2=4Q1�����������H2��ת����Ϊ20%��