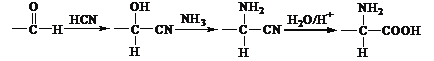
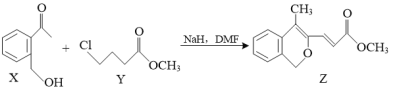��Ŀ����
����Ŀ�����������ϣ�����ִ���ѧ��������ѧʵ����Բⶨ�л������ɺͽṹ��ʵ����ȤС��������ͼ��ʾ��װ�òⶨij�л���X(��Ԫ��C��H��O�е����ֻ�����)����ɡ�ʵ������ƷX���ĵ�����Ϊ1.50g��������þ��������0.90g����ʯ����������2.20g��

(1)��ʵ�����ݷ�������ɵ�X��ʵ��ʽΪ_____��
(2)��������ײ��X�к�����C��O������C��O��C���Ľṹ���������X����Է���������60����X������Ϊ_______��
(3)��ʵ������ͬ״���£�X�������ܶ���H2�ܶȵ�45��(��֪��ͬ״���£�������ܶȱȵ���Ħ������֮��)��X�ܷ���������Ӧ��1mol X�������2mol Na������Ӧ����X�Ľṹ��ʽΪ_____��
���𰸡�CH2O ������� HOCH2CH(OH)CHO
��������
��ʵ�����ݷ�������X��C��H��O�����ʵ����������ó�ʵ��ʽ����������ײ��X�к�����C��O������C��O��C���Ľṹ���������X����Է���������60����д�ṹ��ʽ���ٶ��л�����������������ܶȼ���X����Է��������������л���������ƶ��л��ﺬ�еĹ����ţ����������Ϣȷ���ṹ��ʽ��
(1)�ɸ�����þ��������0.90g����ˮ������Ϊ0.90g������Ԫ�ص����ʵ���Ϊ2��![]() =0.1mol����ʯ����������2.20g����������̼������Ϊ2.20g����̼Ԫ�ص����ʵ���Ϊ
=0.1mol����ʯ����������2.20g����������̼������Ϊ2.20g����̼Ԫ�ص����ʵ���Ϊ![]() =0.05mol�����л�������Ԫ�ص����ʵ���Ϊ
=0.05mol�����л�������Ԫ�ص����ʵ���Ϊ![]() =0.05mol����n(H)��n(C)��n(O)=0.1��0.05��0.05=2��1��1���ɵ�X��ʵ��ʽΪCH2O��
=0.05mol����n(H)��n(C)��n(O)=0.1��0.05��0.05=2��1��1���ɵ�X��ʵ��ʽΪCH2O��
(2)����ʵ��ʽΪCH2O����ΪX�����ʽ����X��̼ԭ�Ӹ���Ϊx�����л���ķ���ʽΪCxH2xOx����������12x+2x+16x=60�����x=2�������ʽΪC2H4O2��������ײ��X�к�����C��O������C��O��C���Ľṹ����X�Ľṹ��ʽΪHCOO CH3�����л�������Ϊ���������
(3)���������ܶ���H2�ܶȵ�45������X����Է�������Ϊ45��2=90����X��̼ԭ�Ӹ���Ϊx�����л���ķ���ʽΪCxH2xOx����������12x+2x+16x=90�����x=3�������ʽΪC3H6O3��X�ܷ���������Ӧ�����л���ṹ�к���ȩ����1mol X�������2mol Na������Ӧ������ӽṹ�к��������ǻ�����X�Ľṹ��ʽΪHOCH2CH(OH)CHO��

����Ŀ����ֹ2020��4��5�գ�ȫ������״��������ȷ�ﳬ��120���������¶�Ԥ���¹ڲ�������ʶ�������Ͽ�ѧ�������ǣ� ��
A | B | C | D |
|
|
|
|
84����Һʹ��ʱ���ܺͽ������� | ҽ�þƾ�����Ч��Ũ��95%>75% | ���ֹؼ�һ��۱�ϩ���粼�����л��߷��Ӳ��� | �¶ȼ���ˮ�����ڽ������� |
A.AB.BC.CD.D
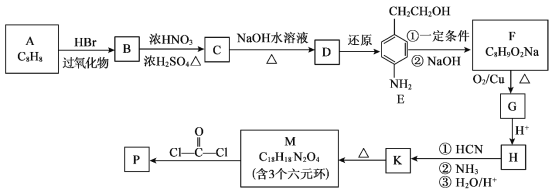
����Ŀ��ij�о���ѧϰС���ͬѧ�������ͼװ����ȡ�屽�������飺

��֪���Ҵ��ڼ��ȵ������¿���HBr��Ӧ�õ������飨CH3CH2Br��������ijЩ�����������±���ʾ��
�ܽ��ԣ������������ܼ��� | �е㣨�棩 | �ܶȣ�g/mL�� | |
�Ҵ� | ��ˮ���ܣ��������л��ܼ� | 78.5 | 0.8 |
������ | ������ˮ���������л��ܼ� | 38.4 | 1.4 |
��ش��������⣺
��1�� B�з�����Ӧ����Ŀ�����Ļ�ѧ����ʽΪ_________��
��2������ʵ��Ŀ�ģ�ѡ�����к��ʵ�ʵ�鲽�裺�١�___________��ѡ��ڢۢܵȣ���
����װ��װ�ã�___________����дʵ��������ƣ���
�ڽ�Aװ���еĴ���˿С�����²��뱽��Һ��Ļ��Һ�У�
�۵�ȼBװ���еľƾ��ƣ���С������ƿ����10���ӣ�
������ƿ�м���һ��������Һ�壬����ƿ�м�����ˮ�Ҵ����Ը��ڽ������ܿڴ�����U�ι��м�������ˮ��ס�ܵף���ˮ���м����ˮ��
��3������ʵ�����ô���˿�������۵��ŵ㣺_____��
��4����ˮ��������_______��
��5����Ӧ��Ϻ�U�ι��ڵ�������______________������������ʱ����IJ���������_____��