��Ŀ����
 ��﮻�ʯ����Ҫ�ɷ���Li2O��Al2O3��4SiO2��������FeO��CaO��MgO�ȡ�
��﮻�ʯ����Ҫ�ɷ���Li2O��Al2O3��4SiO2��������FeO��CaO��MgO�ȡ���
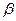 ��﮻�ʯΪԭ���Ʊ�̼��﮵�һ���������£�
��﮻�ʯΪԭ���Ʊ�̼��﮵�һ���������£�
��֪���ٲ��ֽ����������↑ʼ��������ȫ������pH��
| �������� | Fe(OH)3 | Al(OH)3 | Mg(OH)2 |
| ��ʼ����pH | 2.7 | 3.7 | 9.6 |
| ��ȫ����pH | 3.7 | 4.7 | 11 |
| �¶�/�� | 0 | 10 | 20 | 50 | 75 | 100 |
| Li2CO3���ܽ��/g | 1.539 | 1.406 | 1.329 | 1.181 | 0.866 | 0.728 |
��1����ӦII�м�̼��Ƶ������� ��
��2��д����ӦIII�����ɳ���A�����ӷ���ʽ �� ��
��3��д����ӦIV�Ļ�ѧ����ʽ ��ϴ������Li2CO3����Ҫ����ˮ������ˮ��ԭ���� ��
��4��ʵ�����г���������Ũ���Ĺ������ʵ�������______________��
| A�������� | B�������� | C������̨������Ȧ�� | D���ƾ��� E��Բ����ƿ |
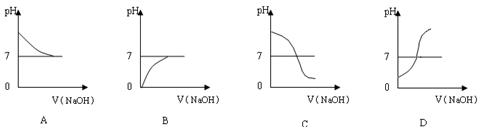
��1����ȥ��Ӧ���й��������ᣬ����pHʹFe 3+��Al3+��ȫ������3�֣�
��2��Mg2++2OH-= Mg(OH)2����2�֣� Ca2++CO32-=CaCO3����2�֣�
��3��Li2SO4+Na2CO3=Li2CO3��+Na2SO4��3�֣�
̼����ڽϸ��¶����ܽ��С������ˮϴ�ӿɼ���̼��﮵���ģ�3�֣�
��4��ABD��3�֣�
��

��ϰ��ϵ�д�
�����Ŀ
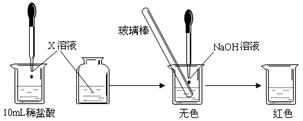
 ��CO
��CO ��OH-�е�������ɣ����Ǿ����������ʣ���A������ˮ�������B������ˮ�����������ᣬ���ų���ɫ�̼�����ζ������E����C��ˮ��Һ�ʼ��ԣ������ᷴӦ����A����D������ˮ������������ʱ�ų�����E��E��ʹ����ʯ��ˮ����ǡ���ش�
��OH-�е�������ɣ����Ǿ����������ʣ���A������ˮ�������B������ˮ�����������ᣬ���ų���ɫ�̼�����ζ������E����C��ˮ��Һ�ʼ��ԣ������ᷴӦ����A����D������ˮ������������ʱ�ų�����E��E��ʹ����ʯ��ˮ����ǡ���ش�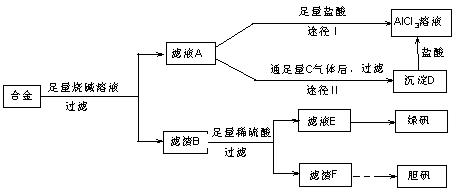
 ��
��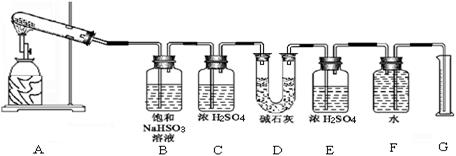
 �������ϴ����������� ��
�������ϴ����������� �� 56
56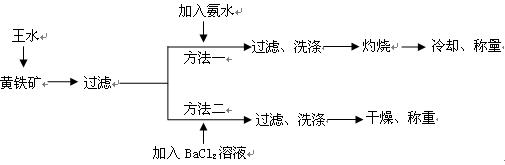
 ��(��=1.42g��cm-3)��3�����Ũ����(��=1.19g��cm-3)��϶��ɵġ�
��(��=1.42g��cm-3)��3�����Ũ����(��=1.19g��cm-3)��϶��ɵġ�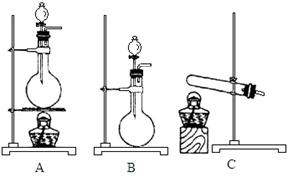
 ���Ʊ������ܴ��Բ��ϵ���Ҫԭ�ϡ�ʵ������
���Ʊ������ܴ��Բ��ϵ���Ҫԭ�ϡ�ʵ������ Ϊԭ���Ʊ������ߴ�
Ϊԭ���Ʊ������ߴ� ��Һ��
��Һ��
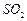 ��
��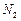 ������壬��Ӧ3h��ֹͣͨ��
������壬��Ӧ3h��ֹͣͨ��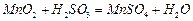 ����
����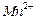 ��
��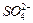 ��Ũ���淴Ӧʱ��t�仯����ͼ��������Һ��
��Ũ���淴Ӧʱ��t�仯����ͼ��������Һ��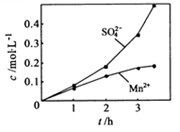
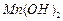 ��ʼ����ʱ
��ʼ����ʱ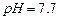 ���벹���ɣ�1���Ƶõ�
���벹���ɣ�1���Ƶõ� ��
��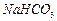 ��
��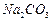 ��
��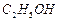 ]��
]��