��Ŀ����
������ˮ��ʯ��ʯ�ķ�Ӧ����ȡ��ŨHClO��Һ�ķ���֮һ��2005��ġ���ѧ����������������ʵ���о���
һ�������о���
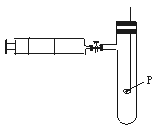
�����Թ��м�������Ŀ�״̼��ƣ��ټ���Լ20 mL������ˮ����ַ�Ӧ�����������ݲ�������Һdz����ɫ��ȥ���ڹ��ˣ�����Һ������ɫ�����ϣ����������ˮ��Ư���Ը�ǿ����Ϊ��ȷ����Ӧ�������Һ��Ϊ���ݣ��ֱ��������ʵ�飺
��һ����ʯ��ˮ��ϣ���������������ɫ������
�ڶ�����ϡ�����ϣ���������������ɫ���壻
�������ݼ��ȣ�������Һ��������д�����ɫ���������
����⣬����ʵ���в�������ɫ�����ΪCO2��
������������⣺
(1)��Ӧ�����õ���ҺƯ������ǿ��ԭ����____________________________________��
(2)��������ʵ�����֪���ڵ���Һ�е����ʳ�CaCl2��HCIO�⣬������_________��
�����������
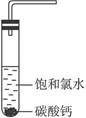
��Բ����ƿ�ײ�����һ����������ס�Ĺ�����״̼��ƺ�150 mL������ˮ������ͼ��ʾװ��ʵ�飬�����ٲ������ݺ���������ʣ���ʯ��ʯ���Һ�棬�ܷ���ټ��ȡ������ƿ�е�Һ�壬ֱ����Ͳ�е����岻�����ӡ�
������������⣺
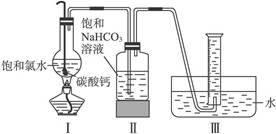
(3)Ϊ������װ�â��ռ����������CO2���ܽ����ɵ���ʧ����ˮ������ȻΪˮ�������װ�â���иĽ�����ķ�����____________________________________________________��
(4)�øĽ����װ�ý�������ʵ�飬����������ڵ�̼�����������A g���ܹ��ռ�����״���µ�CO2����B L������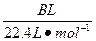 ����С��
����С��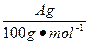 ����������ʵ�������CO2���ܽ��������ɵ����ӻ�ѧ��Ӧԭ��������������һ��С��ϵ��ԭ����____________________________________________��
����������ʵ�������CO2���ܽ��������ɵ����ӻ�ѧ��Ӧԭ��������������һ��С��ϵ��ԭ����____________________________________________��
(5)ʵ����֣�װ�â��е�Һ�������ˣ���ԭ����____________________________________��
һ�������о���

�����Թ��м�������Ŀ�״̼��ƣ��ټ���Լ20 mL������ˮ����ַ�Ӧ�����������ݲ�������Һdz����ɫ��ȥ���ڹ��ˣ�����Һ������ɫ�����ϣ����������ˮ��Ư���Ը�ǿ����Ϊ��ȷ����Ӧ�������Һ��Ϊ���ݣ��ֱ��������ʵ�飺
��һ����ʯ��ˮ��ϣ���������������ɫ������
�ڶ�����ϡ�����ϣ���������������ɫ���壻
�������ݼ��ȣ�������Һ��������д�����ɫ���������
����⣬����ʵ���в�������ɫ�����ΪCO2��
������������⣺
(1)��Ӧ�����õ���ҺƯ������ǿ��ԭ����____________________________________��
(2)��������ʵ�����֪���ڵ���Һ�е����ʳ�CaCl2��HCIO�⣬������_________��
�����������
��Բ����ƿ�ײ�����һ����������ס�Ĺ�����״̼��ƺ�150 mL������ˮ������ͼ��ʾװ��ʵ�飬�����ٲ������ݺ���������ʣ���ʯ��ʯ���Һ�棬�ܷ���ټ��ȡ������ƿ�е�Һ�壬ֱ����Ͳ�е����岻�����ӡ�
������������⣺

(3)Ϊ������װ�â��ռ����������CO2���ܽ����ɵ���ʧ����ˮ������ȻΪˮ�������װ�â���иĽ�����ķ�����____________________________________________________��
(4)�øĽ����װ�ý�������ʵ�飬����������ڵ�̼�����������A g���ܹ��ռ�����״���µ�CO2����B L������
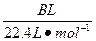 ������
����С��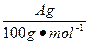 ����������ʵ�������CO2���ܽ��������ɵ����ӻ�ѧ��Ӧԭ��������������һ��С��ϵ��ԭ����____________________________________________��
����������ʵ�������CO2���ܽ��������ɵ����ӻ�ѧ��Ӧԭ��������������һ��С��ϵ��ԭ����____________________________________________��(5)ʵ����֣�װ�â��е�Һ�������ˣ���ԭ����____________________________________��
(1)CaCO3��������ˮ�е�HCl��ʹCl2+H2O HCl+HClOƽ�������ƶ���HClOŨ������
HCl+HClOƽ�������ƶ���HClOŨ������
(2)Ca(HCO3)2
(3)�ڵ���ĩ�������ӳ����ܣ�ʹ���ܵij��ڽӽ���Ͳ�ײ�
(4)̼�����CO2��Ӧ���ɵ�Ca(HCO3)2ʹ����CO2�����ʵ���С��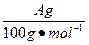
(5)��Բ����ƿ������ˮ�����ڹ��ƿ���������ۼ�
 HCl+HClOƽ�������ƶ���HClOŨ������
HCl+HClOƽ�������ƶ���HClOŨ������(2)Ca(HCO3)2
(3)�ڵ���ĩ�������ӳ����ܣ�ʹ���ܵij��ڽӽ���Ͳ�ײ�
(4)̼�����CO2��Ӧ���ɵ�Ca(HCO3)2ʹ����CO2�����ʵ���С��
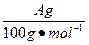
(5)��Բ����ƿ������ˮ�����ڹ��ƿ���������ۼ�
������̽���Ե�ʵ�飬�о����ԺͶ����������ƽ���ƶ���֪ʶ���۲�������������������
����CaCO3��������ˮ�е�HCl��ʹCl2+H2O HCl+HClOƽ�������ƶ���HClOŨ����������̼������ͷų���CO2��ˮ�з�Ӧ����Ca(HCO3)2������
HCl+HClOƽ�������ƶ���HClOŨ����������̼������ͷų���CO2��ˮ�з�Ӧ����Ca(HCO3)2������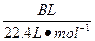 ����С��
����С��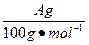 ��ԭ����CO2��û����ȫ�ͷų�ȥ���ɢ����ˮ������ȥ����ô�ڢ�����ȴ�����Ԣ��е�Һ�������ˡ�
��ԭ����CO2��û����ȫ�ͷų�ȥ���ɢ����ˮ������ȥ����ô�ڢ�����ȴ�����Ԣ��е�Һ�������ˡ�
����CaCO3��������ˮ�е�HCl��ʹCl2+H2O
 HCl+HClOƽ�������ƶ���HClOŨ����������̼������ͷų���CO2��ˮ�з�Ӧ����Ca(HCO3)2������
HCl+HClOƽ�������ƶ���HClOŨ����������̼������ͷų���CO2��ˮ�з�Ӧ����Ca(HCO3)2������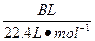 ����С��
����С��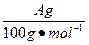 ��ԭ����CO2��û����ȫ�ͷų�ȥ���ɢ����ˮ������ȥ����ô�ڢ�����ȴ�����Ԣ��е�Һ�������ˡ�
��ԭ����CO2��û����ȫ�ͷų�ȥ���ɢ����ˮ������ȥ����ô�ڢ�����ȴ�����Ԣ��е�Һ�������ˡ�
��ϰ��ϵ�д�
 ���ɶ��ȫ���ƿؾ�ϵ�д�
���ɶ��ȫ���ƿؾ�ϵ�д�
�����Ŀ
 HCl+HClO��ƽ���ҪʹHClOŨ�����ӣ��ɼ���
HCl+HClO��ƽ���ҪʹHClOŨ�����ӣ��ɼ���