��Ŀ����
����Ŀ��NaClO2��һ�ָ�Ч��ɱ����������Ҳ������Ư��֯��ȡ�������װ��̽��NaClO2���Ʊ���
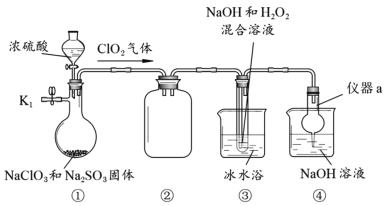
���������գ�
(1)����a������Ϊ__________��װ�âڵ�������________________��
(2)���װ�������Եķ�����________________________________________________��
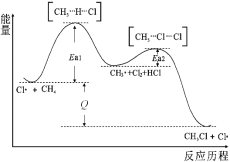
(3)�ر�K1���ӷ�Һ©���м���һ����Ũ���ᣬװ�â�������NaClO2�Ļ�ѧ����ʽΪ2ClO2+2NaOH+H2O2��2NaClO2+2H2O+O2�����÷�Ӧ������������_____________��
(4)ʵ����ɺ�Ϊ��ֹװ���в������ж�������Ⱦ���������Խ��еIJ����ǣ���ֹˮ��K1��____________________________________________��
(5)��װ�â۵���Һ�л��NaClO2�������Ҫ�����м�ѹ����Ũ����________________������ϴ�ӡ�����ȡ�
(6)������NaClO2��3H2O����ʽ���ڣ���֪��NaClO2��3H2O![]() NaCl+O2��+3H2O����Ϊ�ⶨ���ù�����NaClO2��3H2O���������������ռ��������ľ����������ʵ�飺ȡ��Ʒ����Ϊa g�����պ��غõ�����b g��������NaClO2��3H2O������������__________��
NaCl+O2��+3H2O����Ϊ�ⶨ���ù�����NaClO2��3H2O���������������ռ��������ľ����������ʵ�飺ȡ��Ʒ����Ϊa g�����պ��غõ�����b g��������NaClO2��3H2O������������__________��
����������Ӧ4[NaClO2��3H2O]![]() 2NaCl+2NaClO3+O2��+12H2O������ʵ���õ�ֵ��__________����ѡ����ƫ��������ƫС��������������
2NaCl+2NaClO3+O2��+12H2O������ʵ���õ�ֵ��__________����ѡ����ƫ��������ƫС��������������
���𰸡������ ����ȫƿ����ֹ������Һ���������� �ر�K1���رշ�Һ©���������ڢܵ��ձ��м�ˮ��û������¶ˣ���������ƿ�ȣ������г������ݣ�ֹͣ���ȣ����и�����¶���һ��ˮ����һ��ʱ�䲻�䣬������������ O2 ��K1��ͨ����������������������� ���½ᾧ ![]() ��
��![]() ƫС
ƫС
��������
��ʵ��װ��ͼ��֪��װ�âٵ������������Ի����£������ƺ��������Ʒ���������ԭ��Ӧ�Ʊ�ClO2���壻װ�âڵ�����������ȫƿ����ֹ������Һ���������У�װ�â۵��������ڼ��������£�ClO2������������ⷢ��������ԭ��Ӧ�Ʊ�NaClO2��װ�âܵ����������ն����ClO2���壬��ֹ��Ⱦ������
��1������a������Ϊ����ܣ�װ�âڵ�����������ȫƿ����ֹ������Һ���������У��ʴ�Ϊ������ܣ���ȫƿ����ֹ������Һ���������У�
(2)���װ������������Ҫ�γ��ܱ�ϵͳ��Ȼ��ͨ�������¶��γ�ѹǿ���������ǹر�K1���رշ�Һ©���������ڢܵ��ձ��м�ˮ��û������¶ˣ���������ƿ�ȣ������г������ݣ�ֹͣ���ȣ����и�����¶���һ��ˮ����һ��ʱ�䲻�䣬�����������ã��ʴ�Ϊ���ر�K1���رշ�Һ©���������ڢܵ��ձ��м�ˮ��û������¶ˣ���������ƿ�ȣ������г������ݣ�ֹͣ���ȣ����и�����¶���һ��ˮ����һ��ʱ�䲻�䣬�����������ã�
��3���������ѧ����ʽ��֪����Ӧ�й�����������Ԫ�ػ��ϼ����߱�����������������Ӧ�����������Ϊ��ԭ��������Ϊ��������ʴ�Ϊ��O2��
��4��ʵ����ɺ�Ϊ��ֹװ���в�����ClO2������Ⱦ������Ӧ��ֹˮ��K1����K1��ͨ���������������ClO2���������У�������������Һ���գ���ֹ��Ⱦ�������ʴ�Ϊ����K1��ͨ����������������������У�
��5������Һ����ȡ���壬һ���������Ũ�������½ᾧ�����ˡ�ϴ�ӡ�����ķ��������װ�â۵���Һ�л��NaClO2�������Ҫ�����м�ѹ����Ũ�������½ᾧ�����ˡ�ϴ�ӡ�����ʴ�Ϊ�����½ᾧ��
��6���������֪��NaClO2��3H2O���������صõ�NaCl��������ٵ�����Ϊ������ˮ����������������ˮ������Ϊ��a��b���������������ʵ���Ϊxmol���ɷ���ʽ��֪ˮ�����ʵ���Ϊ3xmol����ɵ�32x+18��3x=��a��b�������x=![]() ���ɷ���ʽ�ɵù�ϵʽNaClO2��3H2O��O2������n��NaClO2��3H2O��= n��O2��=
���ɷ���ʽ�ɵù�ϵʽNaClO2��3H2O��O2������n��NaClO2��3H2O��= n��O2��=![]() mol��������NaClO2��3H2O����������Ϊ
mol��������NaClO2��3H2O����������Ϊ![]() =
=![]() ��
��![]() ������������Ӧ4[NaClO2��3H2O]
������������Ӧ4[NaClO2��3H2O]![]() 2NaCl+2NaClO3+O2��+12H2O�����ᵼ�����պ��غ������������b��������
2NaCl+2NaClO3+O2��+12H2O�����ᵼ�����պ��غ������������b��������![]() ��С���ʴ�Ϊ��
��С���ʴ�Ϊ��![]() ��
��![]() ��ƫС��
��ƫС��

����Ŀ��X��Y��Z��W��Ԫ�����ڱ�ǰ�������еij���Ԫ�أ��������Ϣ���±���
Ԫ�� | �����Ϣ |
X | X�Ļ�̬ԭ��L���������K���������2�� |
Y | Y�Ļ�̬ԭ�����������Ų�ʽΪ��nsnnpn+2 |
Z | Z����������Ϊ23��������Ϊ12�ĺ��� |
W | W�ж��ֻ��ϼۣ����ɫ���������ڿ����л�Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ |
��Wλ��Ԫ�����ڱ��� ���ڵ� �壬���̬ԭ��������� �����ӡ�
��X�ĵ縺�Ա�Y�� ��������������С������X��Y����̬�⻯���У����ȶ����� ��д��ѧʽ��
��д��Z2Y2��XY2��Ӧ�Ļ�ѧ����ʽ�����������ת�Ƶķ������Ŀ�� ��
����Xԭ������ԭ���γɵĶ��ַ����У���Щ���ӵĺ˴Ź���������ʾ�������⣬д������һ�ַ��ӵ����ƣ� ����Ԫ�ء�X��Y��ԭ��Ҳ�ɹ�ͬ�γɶ��ַ��Ӻ�ij�ֳ����������ӣ�д������һ�ַ�������������ӷ�Ӧ�����ӷ���ʽ�� ��