��Ŀ����
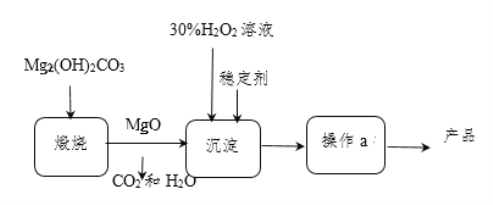
����Ŀ���̷��Ǻ���һ�����ᾧˮ����������,�ڹ�ũҵ�����о�����Ҫ����;��ijͬѧ������������ȡ������������Ҫ�ɷ�ΪFe2O3��SiO2��Al2O3���������������ʣ���ȡ��ˮ������������FeSO4��7H2O�����������ͼ���̣�

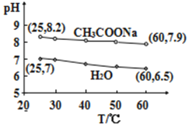
��֪��ijЩ���������ӿ���ͨ��������pH��[��������Һ������ԣ�pH=-lgc(H��), pHֵԽ����Խǿ]ʹ��ת��Ϊ����������2Ϊ��ɫ������
��1����������֮���ʵ�������______________________��
��2���ܽ�����ѡ�õ��������ܷ������ᣬ��˵������___________________________��
��3����֤��Һ2���Ƿ���Fe3+�ķ�����_______________________________________��
��4���Լ�X��������_________________��
��5������Һ2�еõ��̷��IJ�������Ϊ��______________����ȴ�ᾧ�����ˡ���Ȼ�������һϵ�в�����û���õ���������________________�����˿�����ţ�
A.������ B.ʯ���� C.�ձ� D.������
���𰸡����� ����,�����ղ�Ʒ��Ҫ����SO42���������������Cl������ ȡ������Һ2���Թ��У������м���KSCN��Һ������Һ���ɫ��˵����Һ�к� Fe3��������Һ�����ɫ��˵����Һ2�в���Fe3�� ��ԭFe3�� ����Ũ�� B
��������
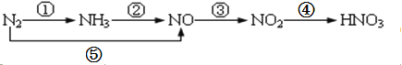
��������Ҫ�ɷ�ΪFe2O3��SiO2 ��Al2O3 ���������������ʣ���ȡ��ˮ������������![]() �����������ᣬFe2O3��Al2O3�����ᷴӦ����
�����������ᣬFe2O3��Al2O3�����ᷴӦ����![]() ��
�� ![]() ��SiO2�������ᷴӦ�dz������������۽�
��SiO2�������ᷴӦ�dz������������۽�![]() ��ԭΪ
��ԭΪ![]() ��
��![]() ����Ӧ����
����Ӧ����![]() ����
����![]() ��
�� ![]() ��
��![]() ��Ӧ����
��Ӧ����![]() ��ɫ������
��ɫ������![]() ��Һ����Ũ������ȴ�ᾧ����������
��Һ����Ũ������ȴ�ᾧ����������![]() ���壬�ݴ˽��
���壬�ݴ˽��
��������������֮��Ӧ���ɹ������Һ������������Һ�IJ����ǹ��ˣ��ʴ�Ϊ�����ˣ�
�ƺ�����ȡ�IJ�Ʒ��![]() ������������������������
������������������������![]() ���ʴ�Ϊ�����ܣ������ղ�Ʒ��Ҫ����
���ʴ�Ϊ�����ܣ������ղ�Ʒ��Ҫ����![]() �������������
�������������![]() ���ʣ�
���ʣ�
�Ǽ���![]() ��
��![]() ��Һ���ʴ�Ϊ��ȡ������Һ2���Թ��У������м���
��Һ���ʴ�Ϊ��ȡ������Һ2���Թ��У������м���![]() ��Һ������Һ���ɫ��˵����Һ�к�
��Һ������Һ���ɫ��˵����Һ�к� ![]() ������Һ�����ɫ��˵����Һ2�в���
������Һ�����ɫ��˵����Һ2�в���![]() ��
��
�Ȳ�Ʒ��![]() ����Ҫ�������۽�
����Ҫ�������۽�![]() ��ԭΪ
��ԭΪ![]() ���ʴ�Ϊ����ԭ
���ʴ�Ϊ����ԭ ![]() ��
��
����ȡ����IJ���һ��������Ũ������ȴ�ᾧ�����ˡ���Ȼ�����Ҫ�õ����������������ձ������������ƾ��ơ�����Ȧ����̨�ȣ��ʴ�Ϊ������Ũ����B��

����Ŀ�����ؽ������ӶԺ������������������Ⱦ��ij��������ˮ��pH=2.0���ѡ�1g��mL-1)�к���Ag+��Pb2+���ؽ������ӣ���Ũ�ȸ�ԼΪ0.01mol��L-1���ŷ�ǰ���ó�������ȥ���������ӣ������й��������£�
���ܵ���� | AgI | AgOH | Ag2S | PbI2 | Pb(OH)2 | PbS |
Ksp | 8.3��10-17 | 5.6��10-8 | 6.3��10-50 | 7.1��10-9 | 1.2��10-15 | 3.4��10-28 |
��1������Ϊ����ˮ��Ͷ��_____������ĸ��ţ�������Ч����á�
A��NaOH B��Na2S C��KI D��Ca(OH)2
��2�������£��������ʯ�Ҵ���������ˮ��ʹ��Һ��pH=8.0��������ķ�ˮ��c(Pb2+)=___��
��3�������ʳ�δ�����ֻ��Ag+�ķ�ˮ����ô�����ķ�ˮ��NaCl����������Ϊ0.117%��������Ҫ���ŷű�Ϊc(Ag+)����1.0��10-8mol��L-1���ʸù���������ķ�ˮ��c(Ag+��=__���Ƿ�����ŷű�__������������������������֪Ksp��AgCl)=1.8��10-10mol2��L-2��
��Ϊ���о������εij����ܽ�ƽ��ͳ���ת����ijͬѧ�������ʵ�顣
����1����2mL0.005 mol/LAgNO3��Һ�м���2 mL0.005 mol/LKSCN��Һ�����á� | ���ְ�ɫ������ |
����2��ȡ1 mL�ϲ���Һ���Թ��У��μ�1��2 mol/LFe(NO3)3��Һ�� | ��Һ��Ϊ��ɫ�� |
����3������2����Һ�У���������5��3mol/LAgNO3��Һ�� | ����a������Һ��ɫ��dz�� |
����4������1���µ���Һ�м���5��3mol/LKI��Һ�� | ���ֻ�ɫ������ |
��֪��25�棬Ksp(AgI����ɫ)=8.3��1017 ��Ksp(AgSCN����ɫ)= 1.0��1012 ��
�ش��������⣺
��4������3������a��_______��
��5����50mL0.005 mol/L��AgNO3��Һ�м���150mL0.005 mol/L��KSCN��Һ������Ϻ���Һ���Ϊ200mL������Һ��Ag+��Ũ��ԼΪ____