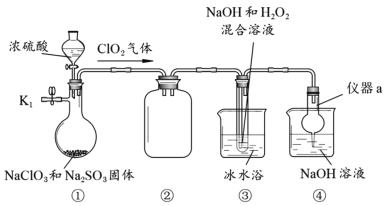
��Ŀ����
����Ŀ��X��Y��Z��W��Ԫ�����ڱ�ǰ�������еij���Ԫ�أ��������Ϣ���±���
Ԫ�� | �����Ϣ |
X | X�Ļ�̬ԭ��L���������K���������2�� |
Y | Y�Ļ�̬ԭ�����������Ų�ʽΪ��nsnnpn+2 |
Z | Z����������Ϊ23��������Ϊ12�ĺ��� |
W | W�ж��ֻ��ϼۣ����ɫ���������ڿ����л�Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ |
��Wλ��Ԫ�����ڱ��� ���ڵ� �壬���̬ԭ��������� �����ӡ�
��X�ĵ縺�Ա�Y�� ��������������С������X��Y����̬�⻯���У����ȶ����� ��д��ѧʽ��
��д��Z2Y2��XY2��Ӧ�Ļ�ѧ����ʽ�����������ת�Ƶķ������Ŀ�� ��
����Xԭ������ԭ���γɵĶ��ַ����У���Щ���ӵĺ˴Ź���������ʾ�������⣬д������һ�ַ��ӵ����ƣ� ����Ԫ�ء�X��Y��ԭ��Ҳ�ɹ�ͬ�γɶ��ַ��Ӻ�ij�ֳ����������ӣ�д������һ�ַ�������������ӷ�Ӧ�����ӷ���ʽ�� ��
���𰸡���4 �� 2 ��С H2O ��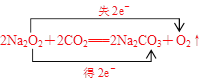
�����飨�����𰸺���Ҳ���֣� CH3COOH��HCO��3=CH3COO����H2O��CO2��
��������
��������Ϣ����֪X��Y��Z��W�ֱ�ΪC��O��n=2����Na������������=������+�������Ƶã���Fe���������������ɰ�ɫ��Ϊ����ɫ���Ϊ���ɫ������Ԫ�ء���Feλ�����ڱ���4���ڵ�����Ԫ�أ����̬ԭ�Ӽ۵����Ų�ʽΪ3d64s2���������2�����ӡ�������ͬһ���ڵ縺���������������ǽ���������ǿ֪X(C)�ĵ縺С��Y(O)��С��X����̬�⻯��CH4û��Y���⻯��H2O�ȶ�����H2O��H2O2�ȶ�����Na2O2��CO2��Ӧ�Ļ�ѧ����ʽΪ2Na2O2��2CO2=2Na2CO3��O2���ڱ����ת�Ƶķ������Ŀʱ��Ӧע��Na2O2����Ԫ�ػ��ϼ�Ϊ-1�ۡ�����С��Ϊ��ɢ�����⣬�𰸲�Ψһ���������к���������ԭ�ӵ����϶࣬����飨CH3CH2CH3���������飨CH3CH2CH2CH3������Ȳ��CH3C��CH���ȣ���C��H��O����Ԫ���γɵķ��Ӻܶ࣬���γɵ���������ֻ��HCO��3��������HCO��3��Ӧ�ķ��ӱ���Ϊ���ᣬ��CH3COOH�ȡ�
���㶨λ��������Ԫ���ƶ���Ϊ���п���ԭ�ӽṹ��Ԫ�����ڱ���Ԫ��������֪ʶ���˴Ź������ס�������ԭ��Ӧ�����ӷ���ʽ����д��ּ�ڿ��鿼����������ɡ��ṹ��������Ԫ�������ڱ��е�λ�õĹ�ϵ���ۺ�Ӧ������������ѧ���ķ�ɢ˼ά������