��Ŀ����
10������A��B��C��D��E��������Һ���ֱ���K+��NH4+��Ag+��Ba2+��Al3+��Cl-��Br-��CO32-��SO42-��NO3-�е��������������Ӹ�һ����ɣ��������������������Ӹ�����ͬ������֪����A+B���ס�����A+C���ס�����A+D���ס�����B+C+H2O���ס�+���壻��A����Һ�����ԣ���B����Һ��c��OH-����c��H+������C��D��E������Һ��pH��7������������ʵ���ش��������⣺
��1��д����ѧʽ��CAl2��SO4��3DAgNO3ENH4Br
��2�������ӷ���ʽ��ʾ��B����Һ��c��OH-����c��H+����ԭ��
���� ��A��B��C��D��E����������Һ����������ˮ��ԭ����֪��ǿ��������ˮ������ԣ�ǿ��������ˮ��ʼ��ԣ�NH4+��Al3+��Ag+Ϊ���������ӣ�K+��Ba2+Ϊǿ�������ӣ�Cl-��Br-��NO3-��SO42-Ϊǿ������ӣ�CO32-Ϊ��������ӣ�����Ag+ֻ��NO3-��ϳ�AgNO3��Һ��Ba2+����SO42-��CO32-���ϣ�AgBrΪdz��ɫ������B����Һ��c��OH-����c��H+����B��Һ�ʼ��ԣ�ֻ����ǿ����������Һ�����Ƶ�ΪK2CO3���ܹ�����˫ˮ�ⷴӦ������Ϊ��2Al3++3CO32-+3H2O�T2Al��OH��3��+CO2����������Ŀ��֪����������⣮
��� �⣺��1����������C��D��E������Һ��pH��7��˵��C��D��E������ҺΪǿ�������Σ�ˮ������ԣ�B��Һ�ʼ��ԣ�A����Һ�����ԣ�Ag+����Cl-��Br-��SO42-��CO32-���ϣ���ΪAgCl��Ag2SO4��Ag2CO3��Ϊ��ɫ������AgBrΪdz��ɫ������ֻ���γ������������Ʋ�֪AΪ������Һֻ���Ȼ���������B����Һ��c��OH-����c��H+����Һ�ʼ��ԣ�BΪǿ�������Σ���K+��Ba2+Ϊǿ�������ӣ�CO32-Ϊ��������ӣ����Bֻ��ΪK2CO3������B��C��Ӧ���ɰ�ɫ���������壬B�к�CO32-��������CO32-��Ӧ���ɳ�������Al3+��Ag+��Ba2+������CO32-��Al3+����˫ˮ�ⷴӦ�������ɳ��������������壬���ӷ�ӦΪ2Al3++3CO32-+3H2O�T2Al��OH��3��+CO2��������C�к�Al3+����CΪǿ��������������Һ���������ΪCl-��Br-��SO42-�е�һ�֣���Ϊ������������A�ɷֱ���B��D��Ӧ���ɰ�ɫ������BΪK2CO3��CΪ��������D��ֻ������������EΪNH4Br��
�ʴ�Ϊ��Al2��SO4��3��AgNO3��NH4Br��
��2��BΪK2CO3����Һ��CO32-����ˮ�ⷴӦ����CO32-+H2O?HCO3-+OH-��ʹ��Һ�ʼ��ԣ�����c��OH-����c��H+����
����Һ��CO32-����ˮ�ⷴӦ����CO32-H2O?HCO+OH-��ʹ��Һ�ʼ��ԣ�����c��OH-����c��H+����
���� ���⿼������ˮ�⡢˫ˮ�⡢���ӷ���ʽ�����ӷ�Ӧ�����ݣ������Ŀ�е���Ϣ����Һ�ʼ��ԡ����ɰ�ɫ������������ǽ���ؼ���ͬʱǰ����ϵ�����ھ������������Ϣ�����ܼ������������⣬��Ŀ�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| ���� | Cu��OH��2 | Fe��OH��3 | CuCl | CuI |
| Ksp | 2.2��10-20 | 2.6��10-39 | 1.7��10-7 | 1.3��10-12 |
��2�����˺�������Һ��������Ũ������ȴ�ᾧ�������ɵõ�CuCl2•2H2O���壮
��3����CuCl2•2H2O����õ�������ˮCuCl2����Ҫ���еIJ�������HCl�����м��ȣ�
��4��ijѧϰС���á���ӵ��������ⶨ����CuCl2•2H2O�������������������I-������Ӧ�������������ʣ��Ĵ��ȣ��������£�ȡ0.800g��������ˮ���������KI���壬��ַ�Ӧ�����ɰ�ɫ��������0.1000mol/L Na2S2O3����Һ�ζ�������ζ��յ�ʱ������Na2S2O3����Һ40.00mL������֪��I2+2S2O32-�TS4O62-+2I-����
�ٿ�ѡ�õ������ζ�ָʾ�����ζ��յ�������ǵ�������ɫ�����ɫ�Ұ���Ӳ��仯��
��CuCl2��Һ��KI��Ӧ�����ӷ���ʽΪ2Cu2++4I-=2CuI��+I2��
�۸�������CuCl2•2H2O�������ٷ���Ϊ85.5%��
 | IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | �� | |||
| 4 | �� | �� |
��2���������γɵļ����ӵĽṹʾ��ͼ
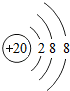 ��
����3���٢ۢ�����Ԫ������������ˮ�����м�����ǿ�����ʵĻ�ѧʽ��NaOH��
��4��Ԫ�آ��γɵ��ȶ����������ں�������й���ʱ�Ļ�ѧ����ʽ�ǣ�2Na2O2+2CO2=2Na2CO3+O2��2Na2O2+2H2O=4NaOH+O2����
��5���ۢݢ�����Ԫ���γɵļ����ӣ����Ӱ뾶�ɴ�С��˳����O2-��Mg2+��Al3+��
��6�����һ����ʵ��֤���������Ԫ�طǽ�����ǿ���ıȽϽ�����ͨ��Na2S��Һ�У������Һ�г���dz��ɫ��������˵�������������Դ�������ǽ�����Cl��S��
| A�� | a-c=m-n | B�� | a-b=n-m | C�� | c+d=m+n | D�� | b-d=n+m |
 ���÷��Ӻ��м��Լ��ͷǼ��Լ�������ԡ������Ǽ��ԡ�����
���÷��Ӻ��м��Լ��ͷǼ��Լ�������ԡ������Ǽ��ԡ����� CaCl2
CaCl2 N2
N2 OH-
OH-
 ��
��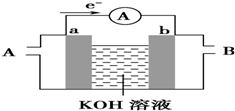 ��1�����������һ�����Ϳɳ���أ�����ͨ�����ȣ��õ���ܽϳ�ʱ�䱣���ȶ��ķŵ��ѹ��������ص��ܷ�ӦΪ��3Zn+2K2FeO4+8H2O $?_{���}^{�ŵ�}$3Zn��OH��2+2Fe��OH��3+4KOH�� ��ش��������⣺
��1�����������һ�����Ϳɳ���أ�����ͨ�����ȣ��õ���ܽϳ�ʱ�䱣���ȶ��ķŵ��ѹ��������ص��ܷ�ӦΪ��3Zn+2K2FeO4+8H2O $?_{���}^{�ŵ�}$3Zn��OH��2+2Fe��OH��3+4KOH�� ��ش��������⣺