��Ŀ����
18������Na2S2O8������ǿ������SO4-•�����ɻ��������л���Ⱦ����Ŀǰ���DZ���ĸ��������������ױ���ij������̽��pH����������Na2S2O8��Ũ�ȶԽ���2��4-DMAP��2��4--�����ӣ�Ч�ʵ�Ӱ�죮��1��̽����Һ����Ե�Ӱ�죺��������2��4-DMAP���뵽��ͬpH��Na2S2O8��Һ�У������ͼ1��ʾ���ɴ˿�֪����Һ������ǿ�������� ��������ڡ������ڡ���Na2S2O8����SO4-•��
��2��̽������������Ӱ�죺
��ʵ��ǰ������0.1mol•L-1 H2SO4ϴ��Fe�ۣ���Ŀ����ȥ�����۱��������������ʣ���������ˮϴ�������ԣ�
����ͬ�����£�ȡ��ͬ������������۷ֱ����c��2��4-DMAP��=1.0��10 -3mol•L-1��c��Na2S2O8��=4.2��10-3mol•L-1�Ļ����Һ�У�ʵ������ͼ2��ʾ�����н���Ч����õ�����������1.5g/L������ӷ�Ӧ��ʼ��A��ʱ��2��4-DMAP�����ƽ����Ӧ����Ϊ2.0��10-5mol/��L•min�������Է�Ӧ��������Һ�������仯����
����֪��2Fe+O2+2H2O=2Fe2++4OH-��S2O8-+Fe2+=SO4-+SO42-+Fe3+������ܻᷢ����SO4-+Fe2+=SO42-+Fe3+�������ۼ���������ʱ��2��4-DMAP�Ľ����ʷ����½���ԭ������ǣ������ӷ���ʽ��ʾ����Fe+2Fe3+=3Fe2+��SO4-+Fe2+=SO42-+Fe3+��
��3��̽��Na2S2O8Ũ�ȵ�Ӱ�죺��д�±��пո����ʵ�鷽����
| ��š������� | M��Fe�ۣ�/g | V[0.05mol��L-1Na2S2O8]/mL | V[H2O]/mL | �ռ����ݺ͵ó����� | |
| 1 | 100 | 0.3 | 50 | 50 | |
| 2 | 100V����ˮ��/mL | 0.3 |
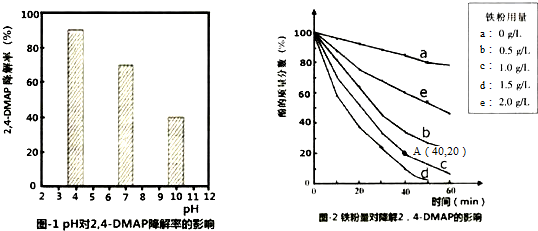
���� ��1��������������ͬ����4-CP���뵽��ͬpH��Na2S2O8��Һ�У�����ͼ��֪����Һ��pHԽС��4-CP������Խ��
��2�����������������������ɳ�ȥ��
�ھ�ͼ����������Ч����õ�����������d���ߣ���1.5gʱ��c��2��4-DMAP��=1.0��10 -3mol•L-1������1L��Һ������2��4--�����ӵ�����Ϊ1.0��10 -3mol•L-1��122g/mol=0.122g�����A��ʱ������������20%���㷴Ӧ���ʣ�
��Fe�ܹ��������ӷ�Ӧ�����������ӣ����������ܹ���ǿ������SO4-•�����ɻ�����ԭ��
��3��̽��Na2S2O8Ũ�ȵ�Ӱ�죬Ӧʹ��������Ũ�Ⱥ�������ͬ��Na2S2O8Ũ�Ȳ�ͬ���۲�����ͬʱ����2��4-DMAP��Ũ�ȱ仯��
��� �⣺��1��������������ͬ����4-CP���뵽��ͬpH��Na2S2O8��Һ�У�����ͼ��֪����Һ��pHԽС��4-CP������Խ��������Һ������ǿ������Na2S2O8����SO4-•���ʴ�Ϊ�������ڣ�
��2��H2SO4���Գ�ȥFe�۱���������
�ʴ�Ϊ��ȥ�����۱��������������ʣ�
�ھ�ͼ����������Ч����õ�����������d���ߣ���1.5g/Lʱ��������1L��Һ������2��4-�������ӵ�����Ϊ1.0��10 -3mol•L-1��122g/mol=0.122g��A��ʱ������80%��Ϊ0.0976g�����ʵ���Ϊ0.0008mol������40minʱ�ķ�Ӧ����Ϊ$\frac{\frac{0.0008mol}{1L}}{40min}$=2.0��10-5mol/��L•min�����ʴ�Ϊ��1.5��2.0��10-5mol/��L•min����
��Fe�ܹ��������ӷ�Ӧ�����������ӣ����������ܹ���ǿ������SO4-•�����ɻ�����ԭ����Ӧ�����ӷ���ʽΪFe+2Fe3+=3Fe2+��SO4-+Fe2+=SO42-+Fe3+��
��3��̽��Na2S2O8Ũ�ȶԶԽ���2��4-DMAP��2��4--�����ӣ�Ч�ʵ�Ӱ�죬Ӧʹ��������Ũ�Ⱥ�������ͬ��Na2S2O8Ũ�Ȳ�ͬ���۲�����ͬʱ����2��4-DMAP��Ũ�ȱ仯�����Լ������50mL��0.05mol��L-1Na2S2O8��Һ��������50mL��0.05mol��L-1Na2S2O8��Һ������Һ�����ҪΪ100mL����Na2S2O8��Һ��ˮ�����֮��Ϊ100mL������30mLNa2S2O8��Һ��70mLˮ���ռ������ݺ͵ó��Ľ����ǣ�����ͬʱ����2��4-DMAP��Ũ�ȱ仯��ͬ����˵��Na2S2O8Ũ�ȶ�2��4-DMAP��������Ӱ�죬��2��4-DMAP��Ũ�ȱ仯����ͬ����˵��Na2S2O8Ũ�ȶ�2��4-DMAP��������Ӱ�죬�ʴ�Ϊ��
| ��š������� | M��Fe�ۣ�/g | V[0.05mol��L-1Na2S2O8]/mL | V[H2O]/mL | �ռ����ݺ͵ó����� | |
| 1 | ����ͬʱ����2��4-DMAP��Ũ�ȱ仯��ͬ����˵��Na2S2O8Ũ�ȶ�2��4-DMAP��������Ӱ�죬��2��4-DMAP��Ũ�ȱ仯����ͬ����˵��Na2S2O8Ũ�ȶ�2��4-DMAP��������Ӱ�죮 | ||||
| 2 | 30 | 70 |
���� ���⿼��̽��ʵ�飬���ؿ���ѧ����ȡ��Ϣ���ӹ���Ϣ��������Ϣ�������������֪��ͼ�����ݺ�����ĺ��弰�仯���ƣ�֪��ʵ��Ŀ�ļ�ԭ������Ŀ�ѶȲ���

�Լ���A����ˮ��B��NaOH��Һ ��C��CaO D������KMnO4 ��Һ E������Na2CO3��Һ
����������a������ b����Һ c��ϴ�� d������ e������ f������ g���ѻ�
| ��� | ���� | ���� | �Լ� | �������� |
| 1 | ���� | ��ϩ | ||
| 2 | �屽 | �� | ||
| 3 | �������� | ���� | ||
| 4 | �Ҵ� | ˮ | ||
| 5 | ������ת��Ϊ���� | |||
| 6 | ��úת��Ϊ��¯����ú���ͺͽ�̿�� | |||
| A�� | �赥�ʹ㷺�����������ά | |
| B�� | SO2ʹ��ˮ��ɫ��˵��SO2����Ư���� | |
| C�� | ����й©��Ӧ˳����ʹ��� | |
| D�� | �����pHֵС��5.6 |
| A�� | ��С | B�� | ������ | C�� | �ȱ���С | D�� | �ȱ�С������ |
| A�� | H2��N2��CCl4 | B�� | CH4��NH3��H2O | C�� | CO2��CS2��Cl2 | D�� | HCl��NO��Br2 |
 ijУ��ѧ��ȤС��Ϊ̽��FeSO4��NaHCO3�ķ�Ӧ������ͼ��ʾ������NaHCO3��Һ�μӵ�FeSO4��Һ�У�FeSO4��NaHCO3��Һ���þ���к���ȴ������ˮ���ƣ�����FeSO4��Һ�м����������ۣ����۲쵽�Թ����������ְ�ɫ������ͬʱ�д�����ɫ�������ɣ�
ijУ��ѧ��ȤС��Ϊ̽��FeSO4��NaHCO3�ķ�Ӧ������ͼ��ʾ������NaHCO3��Һ�μӵ�FeSO4��Һ�У�FeSO4��NaHCO3��Һ���þ���к���ȴ������ˮ���ƣ�����FeSO4��Һ�м����������ۣ����۲쵽�Թ����������ְ�ɫ������ͬʱ�д�����ɫ�������ɣ�
 ��
��
 ��
�� ��
��
 ��F
��F
 +O2$��_{��}^{Cu}$2
+O2$��_{��}^{Cu}$2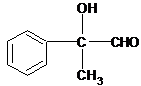 +2H2O
+2H2O +Cu2O��+2H2O
+Cu2O��+2H2O ��
��