��Ŀ����
19���������岻��ȱ����Ԫ�أ�Ϊ�˷�ֹ��ȱ���������г�������һ�ּӵ��Σ������ھ���������һ������KIO3��ȥ��ij�о�С��Ϊ�˼��ij�ӵ������Ƿ��е⣬�������йص����ϣ���������ԭ���ǣ�KIO3+5KI+3H2SO4=3I2+3H2O+3K2SO4��1���÷�Ӧ���������뻹ԭ������������5��1
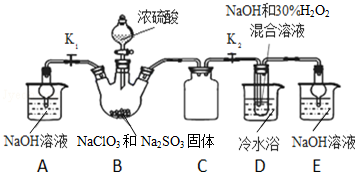
��2����ȡ�����ļӵ��μ�����ˮ�ܽ⣬Ȼ�����ϡ�����KI��Һ��������һ������CCl4������ʱ�۲쵽����������Һ�ֳ����㣬�ϲ���ɫ���²���ϣ��죩ɫ��
��3��ijѧ����һ�η�Һ�����з������²���Һ������ɫҺ�壬��֪����Һ©���е�Һ����һ�����л��㣬��һ����ˮ�㣬�����üķ����������������д���йز��輰�жϷ����ӷ�Һ©���¿ڷų������²�Һ�����С�Թܣ������Թ��м�������ˮ�����Թ���Һ��ֲ㣬��֤���²����л��㣻�����ֲ㣬��֤���²���ˮ�㣻��
��4��������ƿ��ʹ�÷����У����в�����ȷ����AD
A��ʹ������ƿǰ�����Ƿ�©ˮ
B������ƿ��ˮϴ�������ô�����Һϴ��
C��������Һʱ����������Һ�壬����Ͳȡ�����ò�����������������ƿ�У�������ˮ���̶���1��2cm�����ý�ͷ�ιܼ�����ˮ���̶��ߣ�
D���Ǻ�ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת��Σ�ҡ�ȣ�
��5������ʵ�����õ�һ�����ʵ���Ũ�ȵ�ϡ���ᣬ������0.5mol/L��������Һ450mL��������Ͳ��ȡ��������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ��������Ϊ13.6 mL�����ʵ������15mL��20mL��50mL��Ͳ��Ӧѡ��15 mL��Ͳ��ã�
��6����������������ϡ������ҺŨ��ƫ�ߵ���ACD
A���ܽ��ʱ����Һû����ȴ�����¾�ת��
B��ת��ʱû��ϴ���ձ���������
C��������ƿ��ˮ����ʱ�۾�����Һ��
D������Ͳ��ȡŨ�����ϴ����Ͳ����ϴ��Һת�Ƶ�����ƿ
E��ҡ�Ⱥ���Һ����ڿ̶��ߣ��ּ�����ˮ���̶��ߣ�
���� ��1��KIO3+5KI+3H2SO4��3I2+3K2SO4+3H2O�У�IԪ�صĻ��ϼ���+5�۽���Ϊ0��IԪ�صĻ��ϼ���-1������Ϊ0�����ϼ�����ֵ=���ϼ۽���ֵ=ת�Ƶ����������ݻ��ϼ۱仯ȷ������ת�������
��2�����������л��ܼ��������Ȼ�̼�ڵ��ܽ��Զ������ˮ�У��������Ȼ�̼��ȡˮ�еĵ⣬���Ȼ�̼��ˮ�����ܣ���Һ�ֳ����㣬���Ȼ�̼���ܶȱ�ˮ���л������²㣬���������Ȼ�̼���Ϻ�ɫ���ϲ㼸����ɫ��
��3��������ȡ����ˮ�����ܽ�����ƣ��ӷ�Һ©���¿ڷų������²�Һ�����С�Թ��У������Թ��м�������ˮ�����Թ���Һ��ֲ㣬��֤���²����л��㣻�����ֲ㣬��֤���²���ˮ�㣻
��4����������ƿ��ʹ�÷�����ע���������ش����⣻
��5������ϡ��ǰ�����ʵ����������з��������㣬������Ͳ����ȡ������ѡ��
��6���������������ʵ����ʵ��������Һ�������Ӱ�죬����c=$\frac{n}{V}$������������ҺŨ�ȵ�Ӱ�죮
��� �⣺��1��������ԭ��ӦKIO3+5KI+3H2SO4�T3K2SO4+3I2+3H2O�У�IԪ�صĻ��ϼ���+5�۽���Ϊ0��IԪ�صĻ��ϼ���-1������Ϊ0�����ϼ�����ֵ=���ϼ۽���ֵ=ת�Ƶ�����=5������ת��������£� ��KIΪ��ԭ����+5�۵�IԪ�ر���ԭ����ԭ���������������Ϊ�⣬��ԭ���غ㼰��Ӧ��֪�����ʵ���֮��Ϊ1��5���ʴ�Ϊ��5��1��
��KIΪ��ԭ����+5�۵�IԪ�ر���ԭ����ԭ���������������Ϊ�⣬��ԭ���غ㼰��Ӧ��֪�����ʵ���֮��Ϊ1��5���ʴ�Ϊ��5��1��
��2�����������л��ܼ��������Ȼ�̼�ڵ��ܽ��Զ������ˮ�У��������Ȼ�̼��ȡˮ�еĵ⣬���Ȼ�̼��ˮ�����ܣ���Һ�ֳ����㣬���Ȼ�̼���ܶȱ�ˮ���л������²㣬���������Ȼ�̼���Ϻ�ɫ���ϲ㼸����ɫ���ʴ�Ϊ����Һ�ֳ����㣬�ϲ���ɫ���²���ϣ��죩ɫ��
��3���ӷ�Һ©���¿ڷų������²�Һ�����С�Թ��У���������ˮ�����Թ���Һ��ֲ㣬��֤���²����л��㣻�����ֲ㣬��֤���²���ˮ�㣬
�ʴ�Ϊ���ӷ�Һ©���¿ڷų������²�Һ�����С�Թܣ������Թ��м�������ˮ�����Թ���Һ��ֲ㣬��֤���²����л��㣻�����ֲ㣬��֤���²���ˮ�㣻
��4��A��ʹ������ƿǰ������Ƿ�©ˮ������������Һ��Ũ��������A��ȷ��
B������ƿ������ˮϴ�������ô�����Һϴ�ӣ��������ʵ����ʵ���ƫ��������Һ��Ũ��ƫ�ߣ���B����
C������ƿֻ����������һ��Ũ�ȵ���Һ������������ƿ���ܽ����ʣ���C����
D���Ǻ�ƿ�ǣ���ʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ��ת��ҡ����Σ�Ŀ����ҡ����Һ����D��ȷ��
�ʴ�Ϊ��AD��
��5����Ũ��������ΪVmL��ϡ��ǰ�����ʵ��������䣬��98%��1.84g/cm3V=0.5mol/L��0.50L��98g/mol�����V=13.6mL��
Ϊ��С��Ӧѡ��15mL����Ͳ��
�ʴ�Ϊ��13.6��15��
��6��A��δ��ȴ�����¾ͽ���Һת�Ƶ�����ƿ�����ݣ���Һ��ȴ�����ƫС�����Ƶ���ҺŨ��ƫ�ߣ���A��ȷ��
B��ת��ʱû��ϴ���ձ������������������Ƶ���Һ�����ʵ����ʵ���ƫС�����Ƶ���ҺŨ��ƫ�ͣ���B����
C������ʱ���ӿ̶��ߣ����¼��������ˮ���ƫС�����Ƶ���Һ���ƫС����ҺŨ��ƫ�ߣ���C��ȷ��
D��ϴ����Ͳ�����ʵ����ʵ���ƫ�����Ƶ���ҺŨ��ƫ�ߣ���D��ȷ��
E���ּ�����ˮ���̶��ߣ����Ƶ���Һ���ƫ����ҺŨ��ƫС����E����
�ʴ�Ϊ��ACD��
���� ���⿼����ȡ��������ԭ��Ӧ��һ�����ʵ���Ũ����Һ�������йؼ���ȣ��Ѷ��еȣ��Ƕ���ѧ֪ʶ���ۺ����ã���Ҫѧ���߱���ʵ�Ļ���֪ʶ���ۺ�����֪ʶ�������⡢��������������ѧϰ��ȫ����ջ���֪ʶ��

 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�| A�� | ������� | B�� | ǿ����� | C�� | �ǵ���� | D�� | ����� |
| A�� | ̼̼˫��������ת����ϩ��һ����˳���칹 | |
| B�� | CH2Cl2��ͬ���칹�����֤��CH4����������Ľṹ | |
| C�� | �ڶ��ױ�ֻ��һ�ֽṹ����֤������������˫���Ľ���ṹ | |
| D�� | ��ϩ����Ȳ��ƽ���ͷ��ӿ���֪CH3-CH=C��CH3��-C��C-CH3���������е�̼ԭ�ӹ��� |
| A�� | ��ԭ�ӵ�Ħ��������aNAg/mol | |
| B�� | Wg��ԭ�ӵ����ʵ���һ����$\frac{W}{{a{N_A}}}$mol | |
| C�� | Wg��ԭ���к���$\frac{w}{a}$����ԭ�� | |
| D�� | ����֪��Ϣ�ɵã�NA=$\frac{b}{12}$ |
| A�� | 25% | B�� | 35% | C�� | 75% | D�� | 50% |
| A�� | MgSO4�TMg2++SO42- | B�� | Fe��OH��3�TFe3++3OH- | ||
| C�� | NaHCO3�TNa++HCO3- | D�� | KAl��SO4��2�TK++Al3++2SO42- |