��Ŀ����
����Ŀ����1����ijһ�ݻ�Ϊ2L���ܱ������У�ijһ��Ӧ��A��B��C��D������������ʵ���n(mol)��ʱ��t(min)�ı仯������ͼ��ʾ��
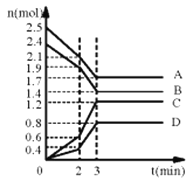
�ش��������⣺
�ٸ÷�Ӧ�Ļ�ѧ����ʽΪ___��
��ǰ2min��A��Ũ�ȱ仯��ʾ�Ļ�ѧ��Ӧ����Ϊ__����2minʱ��ͼ�����ı��ԭ�������__������ĸ����
a.����ѹǿ b.�����¶� c.������� d.����A�����ʵ���
��2����100��ʱ����0.01mol��N2O4�������0.1L���ܱ������з�����Ӧ����һ��ʱ��Ը������ڵ����ʽ��з������õ����±���
ʱ��/s Ũ��/mol��L-1 | 0 | 20 | 40 | 60 | 80 | 100 |
c(N2O4)/mol��L-1 | 0.100 | 0.070 | 0.050 | a | b | c |
c(NO2)/mol��L-1 | 0.000 | 0.060 | d | 0.120 | 0.120 | 0.120 |
����գ�
�ٸ÷�Ӧ�Ļ�ѧ����ʽ__���ﵽƽ��ʱN2O4��ת����Ϊ__��
����0��20s�ڣ�������������ƽ����Ӧ����Ϊ__��
���𰸡�4A+5B6C+4D 0.1mol��L-1��min-1 ac N2O42NO2 60% 0.0015mol��L-1��s-1
��������
(1)�ٸ���ͼ�����ʵ������ӵ�����Ϊ��������ٵ�����Ϊ��Ӧ��仯�����ʵ���֮�ȵ��ڻ�ѧ������֮�ȣ��ó���ѧ����ʽ��
�����û�ѧ��Ӧ���ʹ�ʽv=![]() =
=![]() ���м��㣬����2min������ʵ����仯����ж�Ӱ�����أ�
���м��㣬����2min������ʵ����仯����ж�Ӱ�����أ�
(2)���ݱ����ҵ���Ӧ��������д����ѧ����ʽ�����ݻ�ѧ����ʽ���ó�60sʱ�ﵽƽ�⣬����Ũ�ȵı仯���ֱ����d��a��b��c��ֵ������ת���ʹ�ʽ�����ʹ�ʽ���м��㣻
(1)�ٴﵽƽ��ʱA��B��C��D�ı�����ʵ����ֱ�Ϊ0.8mol��1.0mol��1.8mol��0.8mol����A��B�����ʵ������٣�Ϊ��Ӧ�C��D�����ʵ������ӣ�Ϊ������ʷ�Ӧ�Ļ�ѧ����ʽΪ4A+5B6C+4D��
��ǰ2minʱ�� ����ͼ��2~3minʱͼ���б�ʱ��˵����ѧ��Ӧ���ʱ�죬����ѹǿ���������������ѧ��Ӧ���ʣ��������¶ȼ�С��ѧ��Ӧ���ʣ�����A�����ʵ�������������Ӧ���ʣ���ͼ��б��Ҫͻ�䣬�����ı��ԭ�������ac��
����ͼ��2~3minʱͼ���б�ʱ��˵����ѧ��Ӧ���ʱ�죬����ѹǿ���������������ѧ��Ӧ���ʣ��������¶ȼ�С��ѧ��Ӧ���ʣ�����A�����ʵ�������������Ӧ���ʣ���ͼ��б��Ҫͻ�䣬�����ı��ԭ�������ac��
(2) �ٷ�Ӧ����ʽΪN2O42NO2����ʱ��Ϊ60sʱ��c(NO2)=0.120mol/L���˺�Ũ�Ȳ��ڷ����仯����ʱ��Ϊ60sʱ����Ӧ�ﵽƽ�⡣��N2O42NO2����ã�N2O4�͵�Ũ�ȵı仯��֮��Ϊ1:2��NO2��40sʱ��d=(0.1-0.050)mol/L ��2=0.1 mol/L��a=(0.1-![]() ��0.120)mol/L =0.04 mol/L��60s��NO2��Ũ�Ȳ��ٷ����仯�����60sʱ��Ӧ�ﵽƽ�⣬��b=c=0.04 mol/L��N2O4��ת����Ϊ
��0.120)mol/L =0.04 mol/L��60s��NO2��Ũ�Ȳ��ٷ����仯�����60sʱ��Ӧ�ﵽƽ�⣬��b=c=0.04 mol/L��N2O4��ת����Ϊ![]() ��100%=
��100%=![]() ��100%=60%��
��100%=60%��
����0~20s�ڣ�N2O4��ƽ����Ӧ����Ϊv=![]() =0.0015 mol��L-1��s-1��
=0.0015 mol��L-1��s-1��

 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д� ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д�����Ŀ���Ƚϴ�������ֹ�ҵ�Ʒ�����ȷ����
ѡ�� | ��Ŀ | ��� | �����Ƽ |
A�� | ԭ�� | ʳ�Ρ���������ʯ�� | ʳ�Ρ�������������̼ |
B�� | ���ܵĸ����� | �Ȼ��� | �Ȼ�� |
C�� | ѭ������ | ������������̼ | �������Ȼ��� |
D�� | ���� | ԭ���á����ʸ� | �豸���ܺĵ� |
A.AB.BC.CD.D
����Ŀ����¯���������з�����Ӧ��![]() ���÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ������������˵����ȷ���ǣ� ��
���÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ������������˵����ȷ���ǣ� ��
�¶�T/�� | 1000 | 1150 | 1300 |
ƽ�ⳣ��K | 4.0 | 3.7 | 3.5 |
A.ƽ����¶Ȳ�����С�����ݻ���![]() ��ת��������
��ת��������
B.���Ӹ�¯�ĸ߶ȿ�����Ч��������β����CO�ĺ���
C.������������ʱ������c��CO�����÷�Ӧ��Kֵ����
D.�ɱ������ݿ��жϸ÷�Ӧ����Ӧ�������������������������