��Ŀ����
16���ҹ�ȱ�ⲡ�����㣬����ȱ�ⲡ����Ҫ��ʩ��ʳ���мӵ⣮1996���ҹ������Թ��ұ��ķ�ʽ�涨ʳ�εĵ����Ӽ���KIO3����1�����������ữ��KI���ۻ�������ʳ���Ƿ�Ϊ�ӵ��Σ���Ӧ�Ļ�ѧ����ʽΪKIO3 +5KI+6HCl=6KCl+3H2O+3I2��
��2�����õ绯ѧ�����Ʊ�KIO3��ԭ���ǣ���ʯīΪ�����������Ϊ��������KI��Һ������������Ϊ�������Һ����һ������ǿ�Ⱥ��¶��½��е�⣬�����ܷ�Ӧ����ʽΪKI+3H2O$\frac{\underline{\;���\;}}{\;}$KIO3+3H2������д��������Ӧʽ������I--6e-+6OH-=IO3-+3H2O������6H++6e-=3H2����
��3��������ݻ�Ϊ10L�����ӽ���Ĥ�����У�1minʱ�����ɲ���11.2L����״����Cl2����ʱ��Һ��pH�ǣ������ά�ֲ��䣩13��
��4��Cl2����������ˮ������ɱ��������һ������������ClO2����������ɱ�������еĻ�ԭ�����ΪCl-����������������ˮ������Cl2��ClO2�����ʵ���֮��Ϊ5��2��
���� ��1������KIO3��KI�������ܷ������з�Ӧ�Լ�I2��ʹ���۱�����
��2���ܷ�Ӧ����ʽΪKI+3H2O=KIO3+3H2����IԪ�صĻ��ϼ����ߣ�����������Ӧ�������������ɵ���أ�HԪ�صĻ��ϼ۽��ͣ��������������������Դ������
��3�����ݵ�ⷽ��ʽ��ϲ�������������м���شɣ�
��4�����ݵõ���ͬ��������Ҫ�����ʵ����ʵ��������⣮
��� �⣺��1��KIO3��KI�������ܷ������з�Ӧ������ʽΪ��KIO3 +5KI+6HCl=6KCl+3H2O+3I2���⻯�ر�����������ر���ԭ�������ṩ�ȶ����Ի���������Һ��ʹ��������ɫ����˵��ʳ�������е⣻�ʴ�Ϊ�����ۣ�KIO3 +5KI+6HCl=6KCl+3H2O+3I2��
��2���ܷ�Ӧ����ʽΪKI+3H2O=KIO3+3H2����IԪ�صĻ��ϼ����ߣ�����������Ӧ�������������ɵ���أ�������ӦΪI--6e-+6OH-=IO3-+3H2O��HԪ�صĻ��ϼ۽��ͣ�������������������������ӦΪ6H++6e-=3H2����
�ʴ�Ϊ��I--6e-+6OH-=IO3-+3H2O��6H++6e-=3H2����
��3������Ȼ��Ƶ����ӷ���ʽΪ��2Cl-+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$Cl2��+H2��+2OH-�������ɲ���11.2L����״����Cl2������������Ϊ$\frac{11.2L}{22.4L/mol}$=0.5mol�����ݷ���ʽ����������������1mol�������������Ƶ�Ũ���ǣ�$\frac{1mol}{10L}$=0.1mol/L������������Ũ��Ϊ10-13mol/L����Һ��pH��13���ʴ�Ϊ��13��
��4����һ��ClO2ת���Cl-��Ҫ�õ�10�����ӣ���һ��Cl2ת���Cl-��Ҫ�õ�10�����ӣ���������������ˮ������ת�Ƶ�������ȣ�����Cl2��ClO2�����ʵ���֮��Ϊ5��2���ʴ�Ϊ��5��2��
���� ������Ҫ����ӵ����е�Ԫ�صļ��顢���ԭ����Ӧ���Լ��������仯������й�֪ʶ���ѶȲ��ؼ����յ缫����ʽ����д����Ӧ�õ����غ���м��㣮

 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�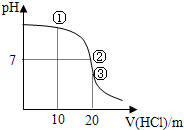
| A�� | ����Һ��c��Cl-����c��NH4+����c��OH-����c��H+�� | |
| B�� | ����Һ��c��NH4+��=c��Cl-��=c��OH-��=c��H��+ | |
| C�� | ����Һ�����е�����Ϊ5�� | |
| D�� | �ζ������п��ܳ��֣�c��NH4+����c��OH-����c��Cl-����c��H+�� |
| A�� | ��ˮ���������c��H+ ��=l.0xl0-12 mol/L | |
| B�� | c ��CH3COOH����c ��H+ ����c ��CH3COO- ����c ��OH-�� | |
| C�� | ��ͬŨ�ȵ�����ֱ��ˮϡ��10����pH �����ᣩ��pH �����ᣩ | |
| D�� | ������������������Һ��Ӧ��c ��CH3COOH��+c ��CH3COO- ��=0.01mol/L�� |
| A�� | ����ԭ�Ӹ�����Ϊ2��3 | B�� | ����Ԫ��������Ϊ1��1 | ||
| C�� | ����Ԫ��������Ϊ5��6 | D�� | ����ԭ�Ӹ�����Ϊ1��1 |
| A�� | ���������ʱ�����ɵ��������������ӵ����� | |
| B�� | ���������ʱ�����ɵ������������������ӵ��Ǽ� | |
| C�� | ���������ʱ�����ɽ��������Ӻ�������ӵ����� | |
| D�� | NH4Cl���� |
| A�� | ԭ�Ӹ���֮��Ϊ1��1 | B�� | ���ʵ���֮��Ϊ1��1 | ||
| C�� | ���֮��Ϊ11��16 | D�� | ������֮��Ϊ1��1 |
| A�� | NO+NO2+2NaOH=2NaNO2+H2O | B�� | 2KClO3$\frac{\underline{MnO_2}}{��}$2KCl+3O2�� | ||
| C�� | CuO+2HNO3�TCu��NO3��2+H2O | D�� | 2FeCl2+Cl2=2FeCl3 |
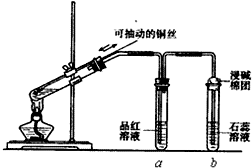 ijͬѧ���ʵ��֤��ͭ��Ũ�����ܷ�����Ӧ��������������������ʣ���ͼ��ʾ�����Թ������2mLŨ���ᣬ�ô����ܺ�һ��С�Ľ����������ӿ��в���һ��ͭ˿�����ȣ��ѷų�����������ͨ��Ʒ����Һ��ʯ����Һ�У�
ijͬѧ���ʵ��֤��ͭ��Ũ�����ܷ�����Ӧ��������������������ʣ���ͼ��ʾ�����Թ������2mLŨ���ᣬ�ô����ܺ�һ��С�Ľ����������ӿ��в���һ��ͭ˿�����ȣ��ѷų�����������ͨ��Ʒ����Һ��ʯ����Һ�У�