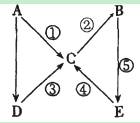
��Ŀ����
��7�֣������±���Ϣ�ش��������⣺���ֶ�����Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼۡ�
(1)E��F��G��Ԫ���γɵĻ������л�ѧ��������______��
(2)B��H��Ԫ�ص��������������Ӧ��ˮ�������Ӧ�����ӷ���ʽ��________��
(3)ʵ��������ȡH���ʷ�Ӧ�Ļ�ѧ����ʽ��________��
(4)��A��B����Ԫ�صĵ����õ������ӽ���NaOH��Һ�У����ֵ������е����������为����ӦʽΪ________��
| Ԫ�� | A | B | C | D |
| ԭ�Ӱ뾶(nm) | 0.130 | 0.118 | 0.090 | 0.102 |
| ��Ҫ���ϼ� | ��2 | ��3 | ��2 | ��6����2 |
| Ԫ�� | E | F | G | H |
| ԭ�Ӱ뾶(nm) | 0.073 | 0.154 | 0.037 | 0.099 |
| ��Ҫ���ϼ� | ��2 | ��1 | ��1 | ��7����1 |
(2)B��H��Ԫ�ص��������������Ӧ��ˮ�������Ӧ�����ӷ���ʽ��________��
(3)ʵ��������ȡH���ʷ�Ӧ�Ļ�ѧ����ʽ��________��
(4)��A��B����Ԫ�صĵ����õ������ӽ���NaOH��Һ�У����ֵ������е����������为����ӦʽΪ________��
(1)���Ӽ����ۼ� (2)Al(OH)3��3H��===Al3����3H2O
(3)MnO2��4HCl(Ũ)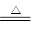 MnCl2��Cl2����2H2O (4)Al��3e����4OH��===AlO2����2H2O
MnCl2��Cl2����2H2O (4)Al��3e����4OH��===AlO2����2H2O
(3)MnO2��4HCl(Ũ)
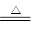 MnCl2��Cl2����2H2O (4)Al��3e����4OH��===AlO2����2H2O
MnCl2��Cl2����2H2O (4)Al��3e����4OH��===AlO2����2H2O����Ԫ�صĽṹ���й����ʿ�֪��A��Mg��B��Al��C��Be��D��S��E��O��F��Na��G��H��H��Cl��
��1��E��F��G��Ԫ���γɵĻ��������������ƣ��������Ӽ��ͼ��Լ���
��2��B��H��Ԫ�ص��������������Ӧ��ˮ����ֱ����������������ᣬ���Զ��߷�Ӧ�ķ���ʽ��Al(OH)3��3H��===Al3����3H2O��
��3��ʵ������ȡ�����Ļ�ѧ����ʽ��MnO2��4HCl(Ũ)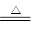 MnCl2��Cl2����2H2O��
MnCl2��Cl2����2H2O��
��4��þ�Ļ�����ǿ�����ģ���þ������������Һ����Ӧ�������ǿ��Է�Ӧ�ģ��������Ǹ�����þ�����������缫��Ӧʽ��Al��3e����4OH��===AlO2����2H2O��
��1��E��F��G��Ԫ���γɵĻ��������������ƣ��������Ӽ��ͼ��Լ���
��2��B��H��Ԫ�ص��������������Ӧ��ˮ����ֱ����������������ᣬ���Զ��߷�Ӧ�ķ���ʽ��Al(OH)3��3H��===Al3����3H2O��
��3��ʵ������ȡ�����Ļ�ѧ����ʽ��MnO2��4HCl(Ũ)
 MnCl2��Cl2����2H2O��
MnCl2��Cl2����2H2O����4��þ�Ļ�����ǿ�����ģ���þ������������Һ����Ӧ�������ǿ��Է�Ӧ�ģ��������Ǹ�����þ�����������缫��Ӧʽ��Al��3e����4OH��===AlO2����2H2O��

��ϰ��ϵ�д�
�����Ŀ
 þ����Ҫ�������£�
þ����Ҫ�������£�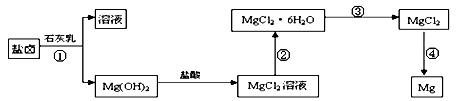
 �ش��������⣺
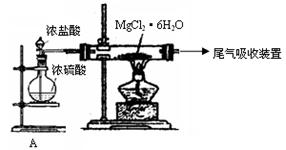
�ش��������⣺ ��
��
 E�������ӽṹʾ��ͼ�� (1��)
E�������ӽṹʾ��ͼ�� (1��) ������ͨ��1L
������ͨ��1L  1mol��L-1
1mol��L-1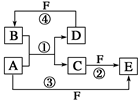
 ________________________________________��
________________________________________�� �ʣ��ҷ�Ӧ����ˮ��Һ�н��С�
�ʣ��ҷ�Ӧ����ˮ��Һ�н��С� �����ֳ���Ԫ��W��X��Y��Z�������γ�һ�ֻ�������������W��Y���������ǵ����������Ҫ���ʣ�X��������ȿ��Ժ�ǿ�ᡢ����Ժ�ǿ�Ӧ��Z�����ש��ɫ�ͺ�ɫ����
�����ֳ���Ԫ��W��X��Y��Z�������γ�һ�ֻ�������������W��Y���������ǵ����������Ҫ���ʣ�X��������ȿ��Ժ�ǿ�ᡢ����Ժ�ǿ�Ӧ��Z�����ש��ɫ�ͺ�ɫ���� �������
������� ���õ���ʽ��ʾ����̬��
���õ���ʽ��ʾ����̬�� ���� ��
���� �� ��
��