��Ŀ����
5��ȡ�ײ�������NaCl�����NaCl������Һ���μ�1-2��Ũ���ᣨԼ12mol/L�������ɹ۲쵽������ɫ��������������������ʵ����ѧ֪ʶ������˵����ȷ���ǣ��������������ij�����NaCl
���Ʋⱥ���Ȼ�����Һ��Ũ��С��12mol/L
�۵õ��Ļ����Һ�д������ܽ�ƽ��
�ܼ���Ũ�����NaCl���ܽ�����С��NaCl�ij�������
�ݼ���Ũ������Ҳ����ʹNaCl������
| A�� | ȫ�� | B�� | �٢ۢ� | C�� | �٢ۢܢ� | D�� | �ڢۢܢ� |
���� ȡ�ײ�������NaCl�����NaCl������Һ����ʱNaCl�Ѿ��ﵽƽ������ܽ�ƽ�⣺NaCl��s��?Na+��aq��+Cl-��aq��������12mol/L��HCl�ɹ۲쵽������ɫ����������˵������������е�������Ũ�ȴ����Ȼ����ܽ�ƽ����������Ũ�ȣ�ƽ�����ƣ��ݴ˽�ɣ�
��� �⣺�ٴ���Һ��ֻ���������ʣ�HCl��NaCl�������Ȼ���Ϊ������Һ�����ƶ������ij�����NaCl������ȷ��
�ڵμ�1-2��Ũ���ᣨԼ12mol/L�������ɹ۲쵽������ɫ���������������Ʋ�ó������Ȼ�����Һ��Ũ��С��12mol/L������ȷ��
�۵õ��Ļ����Һ�д������ܽ�ƽ�⣺NaCl��s��?Na+��aq��+Cl-��aq��������ȷ��
�ܼ���Ũ�����ƽ�����ƣ���NaCl���ܽ�����С��NaCl�ij������ʣ�����ȷ��
�ݼ���Ũ��ʹ����Һ��������С��������ʹ�Ȼ��ƹ���������������Ҳ����ʹNaCl���ܽ�Ƚ�������������ȷ��
��ѡA��
���� ������Ҫ������DZ�����Һ�ĸ���������ܽ�ƽ���Ӱ�����أ��ѶȲ���

��ϰ��ϵ�д�
 ѧҵ����һ��һ��ϵ�д�
ѧҵ����һ��һ��ϵ�д�
�����Ŀ
15��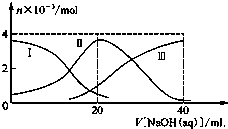 �����£���20mL 0.2mol/L H2A��Һ�еμ�0.2mol/L NaOH��Һ���й��������ʵ����仯��ͼ��ʾ�����Т����H2A�������HA-�������A2-��������ͼʾ�жϣ�����˵����ȷ���ǣ�������
�����£���20mL 0.2mol/L H2A��Һ�еμ�0.2mol/L NaOH��Һ���й��������ʵ����仯��ͼ��ʾ�����Т����H2A�������HA-�������A2-��������ͼʾ�жϣ�����˵����ȷ���ǣ�������
 �����£���20mL 0.2mol/L H2A��Һ�еμ�0.2mol/L NaOH��Һ���й��������ʵ����仯��ͼ��ʾ�����Т����H2A�������HA-�������A2-��������ͼʾ�жϣ�����˵����ȷ���ǣ�������
�����£���20mL 0.2mol/L H2A��Һ�еμ�0.2mol/L NaOH��Һ���й��������ʵ����仯��ͼ��ʾ�����Т����H2A�������HA-�������A2-��������ͼʾ�жϣ�����˵����ȷ���ǣ�������| A�� | ��V[NaOH��aq��]=20 mLʱ����Һ������Ũ�ȴ�С��ϵ��c��Na+����c��HA-����c��H+����c��A2-����c��OH-�� | |
| B�� | �������Ũ�ȵ�NaOH��Һ��H2A��Һ��Ϻ�����Һ��ˮ�ĵ���̶ȱȴ�ˮ�� | |
| C�� | H2A��һ������ķ���ʽΪH2A��HA-+H+ | |
| D�� | ��NaHA��Һ����ˮϡ�͵Ĺ����У�pH��������Ҳ���ܼ��� |
16�������£����и����������ƶ���Һ��һ���ܴ���������ǣ�������
| A�� | ʹ���ȱ��ɫ����Һ��Mg2+��Fe2+��SO42-��NO3- | |
| B�� | ��������Һ��Na+��Ca2+��HCO3-��NO3- | |
| C�� | ʹʯ������ɫ����Һ��Al3+��K+��SO42-��HCO3- | |
| D�� | 0.1mol•L-1NaAlO2��Һ��H+��Na+��Cl-��SO42- |
13����֪�¶�TʱKW=1.0��10-12��0.1mol/L Na2A��ҺpH=6��������˵���У���ȷ���ǣ�������
| A�� | ���¶��£�0.005mol/L H2A��Һ��ˮ�������c��H+��=10-12mol/L | |
| B�� | H2A��ˮ��Һ�еĵ��뷽��ʽΪ��H2A?H++HA-��HA-?A2-+H+ | |
| C�� | ��NH4��2A��Һ�д�������Ũ�ȹ�ϵ����NH4+������A2-������H+������OH-�� | |
| D�� | �������Ũ�ȵ�������H2A��Һ�ֱ���5.6gFe��Ӧ��H2A������H2�� |
20�� �����һ����Ҫ��ˮ����Ⱦ�ij���������õ�ⷨ���ں�Cl-��ˮ���У�̽����NH4+ת��ΪN2���ѵ���Ӱ�����غͷ�Ӧ������
�����һ����Ҫ��ˮ����Ⱦ�ij���������õ�ⷨ���ں�Cl-��ˮ���У�̽����NH4+ת��ΪN2���ѵ���Ӱ�����غͷ�Ӧ������
��1����ⷨ�ѵ���ԭ���������£�
��ֱ�ӵ�����
�ڼ��������£�����2NH3+6OH--6e-=N2+6H2O��Ӧ�ĵ缫Ϊ�������������������������
��•OH�������ɻ���������
�ڵ��������£����ò�����ǿ�������м����•OH�ѵ���•OH��OԪ�صĻ��ϼ�-1��
�ۼ�ӵ�����
���õ�������Cl2����H2O��������HClO�����ѵ�����д��HClO����������������NH4+�����ӷ���ʽ3HClO+2NH4+=N2��+3Cl-+5H++3H2O��
��2��̽�����˵�ʵ������
��ͼΪ��ͬ����ǿ�����ѵ���Ч�����ۺϿ����ܺ����أ�����ǿ��Ӧѡ��10A��
��3���ÿ������һ��̽���ѵ������е�ǿ�����ԵĻ����м�����������¼��裬������ɼ�������
����һ��ֻ��•OH��
�������ֻ��HClO��
������������•OH����HClO��
��4���������ʵ��̽���ѵ��������Ƿ���•OH����������±����ݣ�
��5���о���֪���ѵ�������Ҫ��ԭ����Ϊ������������Һ�б�ǿ����Һ�и�����ʹNH4+ת��ΪN2���ѵ�����ӻ�ѧƽ���ƶ��ĽǶȽ�����ԭ�����Խ�ǿʱ��Cl2+H2O?H++Cl-+HClO�Ļ�ѧƽ�������ƶ������ɵ�HClO���٣������ѵ���
 �����һ����Ҫ��ˮ����Ⱦ�ij���������õ�ⷨ���ں�Cl-��ˮ���У�̽����NH4+ת��ΪN2���ѵ���Ӱ�����غͷ�Ӧ������
�����һ����Ҫ��ˮ����Ⱦ�ij���������õ�ⷨ���ں�Cl-��ˮ���У�̽����NH4+ת��ΪN2���ѵ���Ӱ�����غͷ�Ӧ��������1����ⷨ�ѵ���ԭ���������£�
��ֱ�ӵ�����
�ڼ��������£�����2NH3+6OH--6e-=N2+6H2O��Ӧ�ĵ缫Ϊ�������������������������
��•OH�������ɻ���������
�ڵ��������£����ò�����ǿ�������м����•OH�ѵ���•OH��OԪ�صĻ��ϼ�-1��
�ۼ�ӵ�����
���õ�������Cl2����H2O��������HClO�����ѵ�����д��HClO����������������NH4+�����ӷ���ʽ3HClO+2NH4+=N2��+3Cl-+5H++3H2O��
��2��̽�����˵�ʵ������
��ͼΪ��ͬ����ǿ�����ѵ���Ч�����ۺϿ����ܺ����أ�����ǿ��Ӧѡ��10A��
��3���ÿ������һ��̽���ѵ������е�ǿ�����ԵĻ����м�����������¼��裬������ɼ�������
����һ��ֻ��•OH��
�������ֻ��HClO��
������������•OH����HClO��
��4���������ʵ��̽���ѵ��������Ƿ���•OH����������±����ݣ�
| ʵ�鷽�� | Ԥ��ʵ�����ͽ��� |
| ����һ��pH��NH4+��Cl-Ũ�ȵ���Һ������ѵ���ǿ�ȣ������Ʒ90min���õ��������������Ʒ��•OH |
17��������װ�ý�����Ӧʵ�飬�ܴﵽʵ��Ŀ���ǣ�������
| ѡ�� | A | B | C | D |
| װ�� |  | 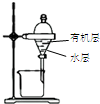 �л���ˮ�� | 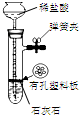 | 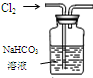 |
| Ŀ�� | ����NH4Cl������Һ�Ʊ�NH4Cl���� | ����CCl4��ȡ��ˮ���ѷֲ���л����ˮ�� | ��ȡ����CO2���� | ��ȥCl2�к��е�����HCl |
| A�� | A | B�� | B | C�� | C | D�� | D |
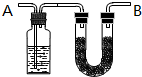 ������C��H��O���л���3.24gװ��Ԫ�ط���װ�ã�ͨ��������O2ʹ֮��ȫȼ�գ������ɵ���������ͨ����A��B�����A������������2.16g��B��������9.24g����֪���л������Է�������Ϊ108��
������C��H��O���л���3.24gװ��Ԫ�ط���װ�ã�ͨ��������O2ʹ֮��ȫȼ�գ������ɵ���������ͨ����A��B�����A������������2.16g��B��������9.24g����֪���л������Է�������Ϊ108�� ��
��