��Ŀ����
 ��һ��ҽҩ�м���,�������Ʊ�����Ѫҩ,��ͨ������·�ߺϳ�
��һ��ҽҩ�м���,�������Ʊ�����Ѫҩ,��ͨ������·�ߺϳ�
��֪:F���G�൱����F������ȥ��1��X���ӡ�
��ش���������:
��1��B��C�ķ�Ӧ������������������������
��2��A��������Һ��Ӧ���������Ļ�ѧ����ʽΪ ����
��3��G�к��еĹ�����Ϊ̼̼˫����������������������;1 mol G ���������������mol NaOH��Ӧ��
��4��G�����������������ֲ�ͬ��������ԭ�ӡ�
��5��д��E��F�Ļ�ѧ����ʽ: ����
��6����������������D��Ϊͬ���칹����л�������������,д����������һ���л���Ľṹ��ʽ:�� ��
��1��ȡ����Ӧ��2�֣�
��2��CH3CHO+2Ag��NH3��2OH H2O+2Ag��+3NH3+CH3COONH4��2�֣�
H2O+2Ag��+3NH3+CH3COONH4��2�֣�
��3���ǻ���1�֣���������1�֣���2��2�֣�����4��6��2�֣�
��5��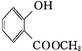 +
+

 +HCl��2�֣�
+HCl��2�֣�
��6��3��2�֣��� ��
�� ��
�� ��1�֣�
��1�֣�
����

 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д��������Ի������Ϊԭ�Ϻϳ�һ���㾫(�������)�ĺϳ�·�ߣ���Ӧ��һ�������½��С�
�ش��������⡣
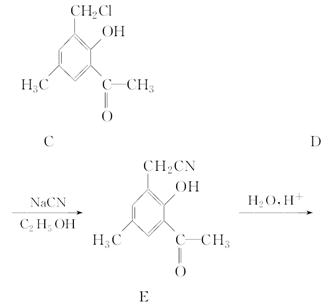
��1������������ɻ������ķ�Ӧ��ԭ�������ʿɴ�100%���������Ľṹ��ʽΪ________________���û�����˴Ź�����������________�����շ塣
��2������������ɻ��������________��Ӧ���仯ѧ����ʽΪ_____________________(ע����Ӧ����)��
��3����������ж���ͬ���칹�塣��д���뻯�����������������ͬ����֧��������ͬ���칹��Ľṹ��ʽ___________________________________________________��
��4������������ܷ����ķ�Ӧ������________(����ĸ)��
| A���ӳɷ�Ӧ | B��������Ӧ | C��ˮ�ⷴӦ | D���Ӿ۷�Ӧ |
 )��һ����Ҫ���л�ԭ�ϣ��ø����ʿɺϳ��������ʡ�
)��һ����Ҫ���л�ԭ�ϣ��ø����ʿɺϳ��������ʡ�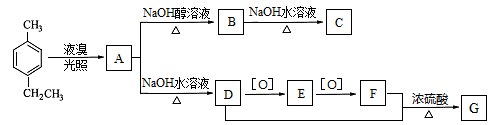
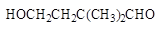 Ϊԭ�ϣ�ijҩ���м���ϳ�·������(���ַ�Ӧ�����Ͳ�����)
Ϊԭ�ϣ�ijҩ���м���ϳ�·������(���ַ�Ӧ�����Ͳ�����)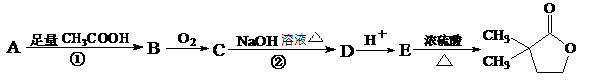




 )��һ����Ҫ�Ļ���ԭ�ϡ���д���Ա�����ȩ���Ҵ�Ϊ��Ҫԭ���Ʊ������������ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�
)��һ����Ҫ�Ļ���ԭ�ϡ���д���Ա�����ȩ���Ҵ�Ϊ��Ҫԭ���Ʊ������������ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�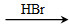 CH3CH2Br
CH3CH2Br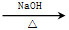 CH3CH2OH
CH3CH2OH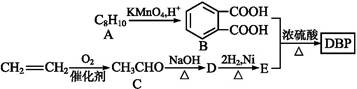
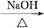 ��
��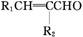 +H2O
+H2O
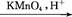
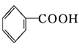
 B
B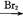 C
C D
D E
E +
+


 +Zn(OH)X
+Zn(OH)X
 G+Zn(OH)Br
G+Zn(OH)Br A+2M(����,F��G��M�ֱ����һ���л���)
A+2M(����,F��G��M�ֱ����һ���л���)
 ��
�� 