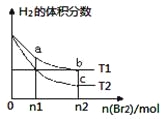
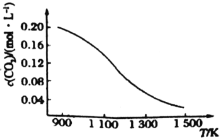
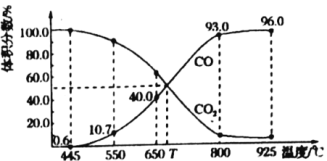
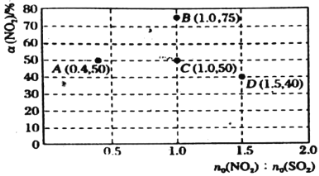
��Ŀ����
����Ŀ��ͭ����Ҫ������Cu���仯�����ڿ�ѧ�о���ҵ�����о���������;����ش��������⣺
��1��CuSO4���ɽ���ͭ��ϡ���Ტͨ��������Ӧ�Ʊ����÷�Ӧ�Ļ�ѧ����ʽΪ_______��
��2����ˮCuSO4��ĩ����������һЩ�л����е���ˮ�֣�������________________��
��3��![]() �����幹����________������Sԭ�ӵ��ӻ����������_______��
�����幹����________������Sԭ�ӵ��ӻ����������_______��
��4��Ԫ�ؽ�Au���������ڱ��еĵ������ڣ���Cuͬ�壬Auԭ�����������Ų�ʽΪ______��һ��ͭ�Ͻ�����������������ܶѻ��Ľṹ���ھ�����Cuԭ�Ӵ������ģ�Auԭ�Ӵ��ڶ���λ�ã��úϽ���ÿһ���Ϊ__________������ò㡱���������ò㡱������ԭ�ӵ���λ��Ϊ__________���þ����У�ԭ��֮�����������________��
��5������������д���ܣ���ԭ�ӿɽ��뵽��Cuԭ����Auԭ�ӹ��ɵ��������϶�С�����Cuԭ����Auԭ�ӵ�ͬ�������þ��崢���ľ����ṹ��CaF2�ľ����ṹ�ṹ���ƣ��þ��崢���Ļ�ѧʽӦΪ___________����Cuԭ����Auԭ�ӵľ���Ϊa cm����þ��崢�����ܶ�Ϊ_________������a�ı���ʽ��
���𰸡�2Cu+O2 +2H2SO4(ϡ) ![]() 2CuSO4 + 2H2O ��ɫ��ĩ�����ɫ���� �������� sp3 6s1 ���ò� 12 ������ H8AuCu3
2CuSO4 + 2H2O ��ɫ��ĩ�����ɫ���� �������� sp3 6s1 ���ò� 12 ������ H8AuCu3 ![]() g/cm3
g/cm3
��������
��1������ͭ��ϡ���Ტͨ��������Ӧ�Ʊ�CuSO4��
��2����ˮCuSO4��ĩΪ��ɫ���壬����ˮ������CuSO4H2O��Ϊ��ɫ���壻
��3�����ݼ۲���ӻ�������ȷ���ӻ���ʽ���ռ乹�ͣ�
��4��AuΪ79��Ԫ�أ�ΪIB��Ԫ�أ�������������������ܶѻ��Ľṹ��ÿһ��Ϊ���ܲ㣻
��5������CaF2�ľ����ṹ�ṹȷ��Hԭ�Ӹ�������ȷ����ѧʽ��
��1������ͭ��ϡ���Ტͨ��������Ӧ�Ʊ�CuSO4������ʽΪ2Cu+O2+2H2SO4(ϡ)![]() 2CuSO4+2H2O��
2CuSO4+2H2O��
��2����ˮCuSO4��ĩΪ��ɫ���壬����ˮ������CuSO4H2O��Ϊ��ɫ���壬����Ϊ��ɫ��ĩ�����ɫ���壻
��3��SO42-����Sԭ�ӹµ��Ӷ���=![]() ��a-bx��=
��a-bx��=![]() ��6+2-2��4��=0��4�����ۼ���Ϊsp3�ӻ������幹��Ϊ�������壻
��6+2-2��4��=0��4�����ۼ���Ϊsp3�ӻ������幹��Ϊ�������壻
��4��AuΪ79��Ԫ�أ�ΪIB��Ԫ�أ������Ϊ�����㣬�����Ų�ʽΪ6s1���Ͻ�����������������ܶѻ��Ľṹ���ھ�����Cuԭ�Ӵ������ģ�Auԭ�Ӵ��ڶ���λ�ã�ÿһ��Ϊ���ܲ㣻��Auԭ�������Cu���������ֱ��ƽ���ϣ�ÿ��������4��������12��������λ��Ϊ12������ԭ��֮����ڽ�������
��5�����崢���ľ����ṹ��CaF2�ľ����ṹ�ṹ���ƣ���Hԭ��λ�ھ������ڲ�����Cuԭ����Auԭ�ӹ��ɵ���������8��������Hԭ�Ӹ���Ϊ8��Cuλ�����ĸ���Ϊ6��![]() =3��Auλ�ڶ������Ϊ8��
=3��Auλ�ڶ������Ϊ8��![]() =1�������ʽΪH8AuCu3��1mol����������=1��8+197+64��3=397g���������ⳤΪl����2l2=��2a��2��l=
=1�������ʽΪH8AuCu3��1mol����������=1��8+197+64��3=397g���������ⳤΪl����2l2=��2a��2��l=![]() a��1mol���������=2
a��1mol���������=2![]() a3NAcm3����=
a3NAcm3����=![]() =
=![]() g/cm3��
g/cm3��

 Ӣ�ŵ��ϵ�д�
Ӣ�ŵ��ϵ�д� ������������Ծ�ϵ�д�
������������Ծ�ϵ�д� �������Ӳ�ϵ�д�
�������Ӳ�ϵ�д�