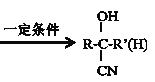
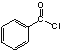
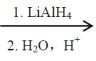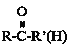��Ŀ����
����Ŀ���״���ˮ�������������ֱ������ȼ�ϵ�ء��ش��������⣺
��1����֪���״��ֽⷴӦ��CH3OH(g)![]() CO(g)��2H2(g) ��H1��________ kJ��mol��1ˮ�����任��Ӧ��CO(g)��H2O(g)
CO(g)��2H2(g) ��H1��________ kJ��mol��1ˮ�����任��Ӧ��CO(g)��H2O(g)![]() CO2(g)��H2(g) ��H2����41��20 kJ��mol��1����CH3OH��g����H2O��g��
CO2(g)��H2(g) ��H2����41��20 kJ��mol��1����CH3OH��g����H2O��g��![]() CO2��g����3H2��g����H3��+49.44kJ��mol��1��
CO2��g����3H2��g����H3��+49.44kJ��mol��1��
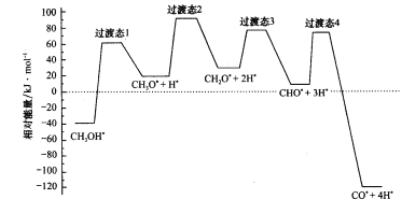
��2����ѧ��ͨ���ܶȷ��������о��״���ˮ�����������ⷴӦ����ʱ���õ��״���Pd�����淢������ʱ�ĸ�·�������������ϵ��ͼ��ʾ�����и���Pd�������������*��ע���������л����С�ķ�Ӧ����ʽΪ_________________________��
��3����0.1MPa�£����ܽ�����1 mol��n��CH3OH����n��H2O����1��1.3�Ļ���������һ�����ܱ������з�Ӧ��
��ʵ����ˮú���任��Ӧ���������¶ȵ����������½���ԭ����__________��
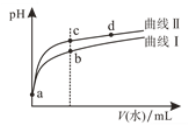
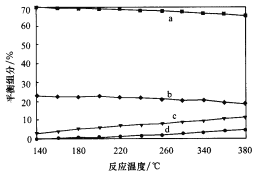
��ƽ��ʱ�����CH3OH�ĺ����ڸ����¶ȷ�Χ�ڼ�С��H2��H2O��g����CO��CO2������ֺ����뷴Ӧ�¶ȹ�ϵ��ͼ��ʾ���Խ���a�ĺ���Լ��c�ĺ���3����ԭ��__________��
��4��297 Kʱ�����ܱ�������(���Ϊ4L���͢����Ϊ8L���зֱ�����������ʷ�����Ӧ��
��� | CO(mol) | H2(mol) | CH3OH(mol) |
�� | 4 | a | 0 |
�� | 4 | 4 | 4 |
��ƽ��ʱ����������c(H2)�����c(H2)=0.5mol/L����
��a=_____________��
�ڸ��¶�ʱ�����з�Ӧ��K=___________��
�ۢ��а��������ݳ��뷴Ӧ���ʱ��Ӧ�ķ���__________���������С�����ƽ�⡱��������С�����
���𰸡�90.64 CH2O*+2H*=CHO*+3H* (��CH2O*=CHO*+H*) ���¶����ߣ��������Խ��� aΪ������cΪCO2��������ͨ����ӦCH3OH��H2O ![]() CO2��3H2 ���ɣ������ĺ����Ƕ�����̼��3�� 6 0.25 ƽ��
CO2��3H2 ���ɣ������ĺ����Ƕ�����̼��3�� 6 0.25 ƽ��
��������
(1)�״��ֽⷴӦ��CH3OH(g) ![]() CO(g)+2H2(g) ��H1�٣�ˮ�����任��Ӧ��CO(g)+H2O(g)
CO(g)+2H2(g) ��H1�٣�ˮ�����任��Ӧ��CO(g)+H2O(g) ![]() CO2(g)+H2(g) ��H2=-41.20 kJ/mol�ڣ�CH3OH(g)+H2O(g)
CO2(g)+H2(g) ��H2=-41.20 kJ/mol�ڣ�CH3OH(g)+H2O(g) ![]() CO2(g)+3H2(g) ��H3��+49.44kJ��mol��1�ۣ����ݸ�˹���ɢ�=��-�ڣ����������H1=49.44kJ��mol��1 -(-41.20 kJ/mol)= +90.64 kJ/mol���ʴ�Ϊ��90.64��
CO2(g)+3H2(g) ��H3��+49.44kJ��mol��1�ۣ����ݸ�˹���ɢ�=��-�ڣ����������H1=49.44kJ��mol��1 -(-41.20 kJ/mol)= +90.64 kJ/mol���ʴ�Ϊ��90.64��
(2)���Ϊ��Ӧ��������������̬����֮���ͼ�п��Կ���������̬3�����ķ�Ӧ�����С����Ӧ��ΪCH2O*+2H*������ΪCHO*+3H���ʷ�Ӧ����ʽΪCH2O*+2H*=CHO*+3H*����Ϊ2H*��Ӧǰ�������ڴ������棬δ���뷴Ӧ���ʷ�Ӧʵ��ΪCH2O*=CHO*+H*���ʴ�Ϊ��CH2O*+2H*=CHO*+3H* (��CH2O*=CHO*+H*)��
(3)����Ϊ�¶����ߣ���Ӧ����Ӧ�ӿ죬��ͼ�����ʼ�С����Ȼ�����¶ȵ�Ӱ�죬ֻ��Ϊ�����Ļ��Խ��ͣ��ʴ�Ϊ�����¶����ߣ������Խ��ͣ�
�ڶ��ڷ�ӦCO(g)+H2O(g) ![]() CO2(g)+H2(g) ��H��0��������������ʱ�������¶ȣ�ƽ�������ƶ�����CO��H2O�ĺ���������CO2��H2�ĺ�������С������ͼ����Ϣ���ɳ�����֪��a��b���߷ֱ��ӦCO2��H2��c��d�������ӦCO��H2O(g)�����ݷ�Ӧ����ʽ��֪���÷�Ӧ��ʼʱ��n(H2)��n(CO2)��n(H2O)��n(CO)��ƽ��ʱ������Ȼ��n(H2)��n(CO2)��n(H2O)��n(CO)����a��b��c��d���߷ֱ��ӦH2��CO2��H2O(g)��CO��a�ĺ���Լ��c�ĺ���3����˵����Ӧ��CH3OH��H2O
CO2(g)+H2(g) ��H��0��������������ʱ�������¶ȣ�ƽ�������ƶ�����CO��H2O�ĺ���������CO2��H2�ĺ�������С������ͼ����Ϣ���ɳ�����֪��a��b���߷ֱ��ӦCO2��H2��c��d�������ӦCO��H2O(g)�����ݷ�Ӧ����ʽ��֪���÷�Ӧ��ʼʱ��n(H2)��n(CO2)��n(H2O)��n(CO)��ƽ��ʱ������Ȼ��n(H2)��n(CO2)��n(H2O)��n(CO)����a��b��c��d���߷ֱ��ӦH2��CO2��H2O(g)��CO��a�ĺ���Լ��c�ĺ���3����˵����Ӧ��CH3OH��H2O![]() CO2��3H2���У��ʴ�Ϊ��a Ϊ������c ΪCO2��������ͨ����ӦCH3OH��H2O
CO2��3H2���У��ʴ�Ϊ��a Ϊ������c ΪCO2��������ͨ����ӦCH3OH��H2O ![]() CO2��3H2 ���ɣ������ĺ����Ƕ�����̼��3����
CO2��3H2 ���ɣ������ĺ����Ƕ�����̼��3����
(4)���ݷ�ӦCH3OH(g)![]() CO(g)��2H2(g)�ٲ���һ�ߵ��������ת��Ϊ8mol CO��12molH2��������Ϊ���һ�룬ƽ��״̬һ������a=12mol/2=6mol���ʴ�Ϊ6��
CO(g)��2H2(g)�ٲ���һ�ߵ��������ת��Ϊ8mol CO��12molH2��������Ϊ���һ�룬ƽ��״̬һ������a=12mol/2=6mol���ʴ�Ϊ6��
���¶���ͬ������K��ȣ����ݢ���㣬ƽ��ʱc(H2)=0.5mol/L��n(H2)=4mol����������ʽ��

��֪����ƽ��״̬��ƽ��ʱc(H2)=0.5mol/L��c(CO)=0.5mol/L��c(CH3OH)=0.5mol/L��K=![]() ���ʴ�Ϊ0.25��
���ʴ�Ϊ0.25��
�������ʿ�֪�����а��������ݳ��뷴Ӧ��ʱ��Ӧ�ķ��䣬�ʴ�Ϊ��ƽ�⡣

����Ŀ�����������(Na2S2O3)��һ�ֽⶾҩ�����ڷ�����顢����Ǧ����������ж����ٴ�����������ݡ���Ƥ�������Ȳ�֢.��������������Ի���Ի������ȶ�����������Һ�зֽ����S��SO2
ʵ��I��Na2S2O3���Ʊ�����ҵ�Ͽ��÷�Ӧ��2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2�Ƶã�ʵ����ģ��ù�ҵ���̵�װ����ͼ��ʾ��

(1)����a��������_______������b��������_______��b��������������Ϊ70%80%��H2SO4��Һ��Na2SO3���巴Ӧ�Ʊ�SO2��Ӧ�Ļ�ѧ����ʽΪ_______��c���Լ�Ϊ_______
(2)ʵ����Ҫ����SO2���������ʣ����Բ�ȡ�Ĵ�ʩ��_______ (д��һ��)
(3)Ϊ�˱�֤��������ƵIJ�����ʵ����ͨ���SO2���ܹ�����ԭ����_______
ʵ���̽��Na2S2O3����������ӵ�������ԭ��Ӧ��
���ϣ�Fe3++3S2O32-Fe(S2O3)33-(�Ϻ�ɫ)
װ�� | �Լ�X | ʵ������ |
| Fe2(SO4)3��Һ | ��Ϻ���Һ�ȱ���Ϻ�ɫ��30s����Ϊ��ɫ |
(4)��������ʵ���������ж�����Fe3+��S2O32-��ԭΪFe2+��ͨ��_______(��������Լ�������)����һ��֤ʵ������Fe2+���ӻ�ѧ��Ӧ���ʺ�ƽ��ĽǶȽ���ʵ��������_______
ʵ��궨Na2S2O3��Һ��Ũ��
(5)��ȡһ�������IJ�Ʒ���Ƴ������������Һ�����ü�ӵ������궨����Һ��Ũ�ȣ��÷�����ƽȷ��ȡ������K2Cr2O7(Ħ������Ϊ294gmol-1)0.5880g��ƽ���ֳ�3�ݣ��ֱ����3����ƿ�У���ˮ�����Һ�������������KI���ữ���������з�Ӧ��6I-+Cr2O72-+14H+ = 3I2+2Cr3++7H2O���ټ��뼸�ε�����Һ������������Na2S2O3��Һ�ζ���������ӦI2+2S2O32- = 2I- + S4O62-���������� Na2S2O3��Һ��ƽ�����Ϊ25.00 mL�������궨�������������Һ��Ũ��Ϊ_______molL-1