��Ŀ����
10���ش��������⣺�١����Ǽ����л�������ƻ�ѧʽ��
��2��2��3-�������顢��3-��-1�����顢�ۼױ�����
 ����ClCH=CHCl��
����ClCH=CHCl�� ����
���� ����
���� ����
����
�ݴ˻ش��������⣺
��1����ϵͳ�����������л����͢��3��3��4-�������飬����������
��2�������л����У���Ϊͬ���칹����Ǣڢߣ��ñ�ű�ʾ������Ϊͬϵ����Ǣٺ͢ࡢ�ۺ͢ᣨ�ñ�ű�ʾ��������˳���칹���Ǣݣ��ñ�ű�ʾ����
��3���۱����ϵĶ��ȴ�����6�֣��Ը��л���Ϊԭ���Ʊ�TNTըҩ�Ļ�ѧ����ʽΪ

��4���������Ǣ���2-����ϩ�������ɣ�д�����Ľṹ��ʽ
 ����
���� ��
�����ݹ����Ŷ������о������ã����Ŷ�������Ӱ�����ã������������
��
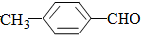

 �ܽ����ɴ�С��˳��
�ܽ����ɴ�С��˳�� ��
�� ��
��
�ڱ����������顢�Ҷ����ķе��ɸߵ��͵�˳����������Ҷ���������
�۱��ӡ������ᡢ�����������ǿ������˳����������
����ʾ����ȷ��봼�����ԣ�
��ij�л�������0.1L��a LO2��һ��������ǡ��ȼ�գ�����0.3Lˮ������0.1LCO2��0.1LCO���������������ͬ�����²�ã���
��1��a�����ֵΪ0.3���л���Ľṹ��ʽΪCH3CH3��
��2�����л���Ϊ�Ҷ���ʱ��a��ֵΪ0.2��
���� ��1����Ϊ��������������������ԭ��Ը��л��������������Ϊ����ͬϵ����ݱ���ͬϵ�����������������ɣ�
��2��ͬϵ���ǽṹ���ƣ���������CH2ԭ���ŵ����ʻ���ͬϵ�ͬ���칹���Ƿ���ʽ��ͬ�ṹ��ͬ�����ʣ�ϩ������˳���칹��
��3���ױ������ϵĶ���ȡ������Կ����ױ���2����ԭ�ӱ�2��Hԭ��ȡ�����ױ���Ũ���������¿���Ũ���ᷴӦ����TNT��
��4�����ݼӾ۷�Ӧԭ���жϷ�Ӧ����Ľṹ��ʽ��
������ȩ����������̼ԭ����Խ�࣬��Ӧ�л�����ܽ��ԽС��
���ǻ���ĿԽ�࣬�л���е�Խ�ߣ����鳣����Ϊ��̬���ݴ��жϸ��л���ķе��С��
�۱�������������˵����Ӱ�죬������������ǿ�����������С�ڱ����
��1���л����в�����ԭ��ʱ����������������ݴ˼����a�����ֵ����ȷ�����л������ɡ��ṹ��ʽ��
��2��������ԭ�������غ�����aֵ��
��� �⣺I����1���� Ϊ�������̼������6��̼��������Ϊ���飬��3��C����2��������4��C����1������������Ϊ��3��3��4-�������飬
Ϊ�������̼������6��̼��������Ϊ���飬��3��C����2��������4��C����1������������Ϊ��3��3��4-�������飬
�� ���Կ���6����ȷ���˱�������Hԭ�ӣ�������Ϊ��������
���Կ���6����ȷ���˱�������Hԭ�ӣ�������Ϊ��������
�ʴ�Ϊ��3��3��4-�������飻��������
��2����3-��-1-��������� ����ʽ��ͬ���ṹ��ͬ��������ͬ���칹�壻
����ʽ��ͬ���ṹ��ͬ��������ͬ���칹�壻
������Ϊ�����������ṹ���ơ�����ʽ��ͬ��������ͬϵ��ۺ͢������1���������ҷ���ʽ��ͬ����Ϊ����ͬϵ�
��Ϊ1��2-������ϩ��̼̼˫���ϵ�C���Ӳ�ͬԭ�ӻ�ԭ���ţ�����˳���칹��
�ʴ�Ϊ���ڢߣ��ٺ͢ࡢ�ۺ͢�ݣ�
��3���ױ���3�ֲ�ͬ��Hԭ�ӣ���һ�ȴ�����3�֣��ֱ�Ϊ��λȡ������λȡ���Ͷ�λȡ�����ڴ˻����ϵó����ȴ���ֱ��У�4�֡�2�֣��ܹ�6�֣��ױ���Ũ���������¿���Ũ���ᷴӦ����TNT����Ӧ�Ļ�ѧ����ʽΪ ��
��
�ʴ�Ϊ��6�� ��
��
��4�������ϩ��2-����ϩ�ж�����C=C���ɷ����Ӿ۷�Ӧ������ʱ��ϩ��C=C���ѣ������µ�C=C����Ӧ����Ϊ�� ��
�� ��
��
�ʴ�Ϊ�� ����
���� ����
����
II��������ȩ���ı��������е�̼ԭ��Խ�࣬��Ӧ�л�����ܽ��ԽС�������ߵ��ܽ�ȴ�СΪ�� ��
�� ��
�� ��
��
�ʴ�Ϊ�� ��
�� ��
�� ��
��
�ں����ǻ���ĿԽ�࣬��ˮ�еķе�Խ�ߣ������ڳ�����Ϊ��̬�������ߵķе��СΪ�����������Ҷ��������飬
�ʴ�Ϊ�����������Ҷ��������飻
���ܱ�����Ӱ�죬����������Դ��ᣬ��������ʯ̼�ᣬ�����������������Դ�СΪ�������������ӣ�
�ʴ�Ϊ�������������ӣ�
��1���л�������0.1L��a LO2��һ��������ǡ��ȼ�գ�����0.3Lˮ������0.1LCO2��0.1LCO����ͬ��������������֮�ȵ������ʵ���֮�ȣ����л�������к���C��Hԭ�����ֱ�Ϊ��N��C��=��$\frac{0.1+0.1}{0.1}$=2��N��H��=$\frac{0.3��2}{0.1}$=6��
���л�������в�����ԭ��ʱ����ȼ��ʱ���ĵ�����������л���ķ���ʽΪ��C2H6��Ϊ���飬�ṹ��ʽΪ��CH3CH3��
������ԭ���غ㣬0.1L����ȼ������0.1L������̼��0.1LCOʱ�������������Ϊ��$\frac{0.1��2+0.1+0.3��1}{2}$L=0.3L��
�ʴ�Ϊ��0.3��CH3CH3��
��2�����л���Ϊ�Ҷ���ʱ�����������غ㶨�ɿɵã�0.1��2+2a=0.1��2+0.1+0.3��1����ã�a=0.2��
�ʴ�Ϊ��0.2��
���� ���⿼�����л���ṹ�����ʣ���Ŀ�Ѷ��еȣ��漰�л����������л������ʽ���ṹ��ʽ��ȷ����ͬ���칹�����д���л���ṹ�����ʵ�֪ʶ������֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ���ѧ���ķ������������Ӧ��������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | ��ɫ��Һ��K+��Al3+��NO3-��HCO3- | |
| B�� | ������c��H+��/c��OH-��=1��10-12����Һ��Na+��K+��AlO2-��CO32- | |
| C�� | ǿ������Һ��NH4+��Na+��SO32-��NO3- | |
| D�� | ���뱽������ɫ����Һ��K+��NH4+��Cl-��I- |
| ������ | ȼ���� | ������ | ȼ���� |
| ���� | 891.0 | ������ | 2878.0 |
| ���� | 1560.8 | �춡�� | 2869.6 |
| ���� | 2221.5 | 2-������ | 3531.3 |
| A�� | ���ȶ��ԣ������飼�춡�� | |
| B�� | ����ȼ�յ��Ȼ�ѧ����ʽΪ�� 2C2H6��g��+7O2��g����4CO2��g��+6H2O��g����H=-1560.8KJ/mol | |
| C�� | �������ȼ���Ȳ�����3530 KJ/mol | |
| D�� | ��ͬ������������̼����������Խ����ȫȼ�շų�������Խ�� |
| A�� | ��ˮ����Ĺ����в�����������ԭ��Ӧ | |
| B�� | ����ұ�������������û���Ӧ�õ������� | |
| C�� | ʯ�ͷ����������仯���ɵõ����͡�ú�ͺͲ��͵� | |
| D�� | CO2��NO2����SO2���ᵼ��������γ� |
| A�� | ���¶��´���ˮ��pD=7 | |
| B�� | ��1L D2O����Һ���ܽ�0.01mol NaOD������Һ���Ϊ1L��������pD=12 | |
| C�� | ��100mL 0.25mol•L-1��DCl��ˮ��Һ�м���50mL 0.2mol•L-1��NaOD����ˮ��Һ������pD=2 | |
| D�� | ��1L D2O���ܽ�0.01mol DCl������Һ�����Ϊ1L��������pD=2 |
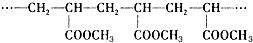
| A�� | �������۷�Ӧ�IJ��� | B�� | �߷��ӵķ���ʽ��C4H6O2 | ||
| C�� | �ϳ����ĵ�����CH2=CHCOOCH3 | D�� | �߷��ӵ���Է���������86 |
| A�� | 2�� | B�� | 3�� | C�� | 4�� | D�� | 5�� |
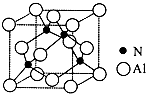 �� ��֪�������ľ����ṹ��ͼ��ʾ����ش��������⣮
�� ��֪�������ľ����ṹ��ͼ��ʾ����ش��������⣮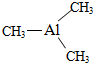 ������
������