��Ŀ����
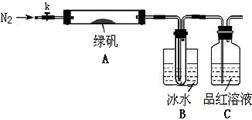
��1�����µ�⼼���ܸ�Чʵ��CO2(g) + H2O(g) ="CO(g)" + H2(g) +O2(g) ������ԭ��ʾ��ͼ���£�

�ٵ缫b���� �����������ԭ������Ӧ��
��CO2�ڵ缫a�ŵ�ķ�Ӧʽ�� ��
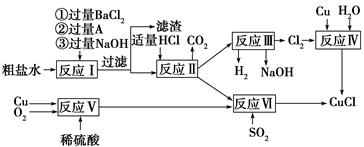
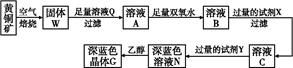
��2����ҵ����ij����������Cu2O��Al2O3��Fe2O3��SiO2����ȡͭ�IJ����������£�
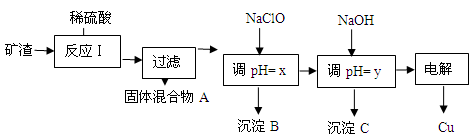
��֪�� Cu2O + 2H+ =" Cu" + Cu2+ + H2O
�ٹ�������A�еijɷ��� ��
�ڷ�Ӧ����ɺ���Ԫ�صĴ�����ʽΪ ���������ӷ��ţ�
��д�����ɸ����ӵ����ӷ���ʽ ��
��x����ֵ��Χ��3.2��pH��4.0��y��Ӧ����ֵ��Χ�� ��
�����й���NaClO��pH��˵����ȷ���� ������ţ���
a������NaClO��ʹ��Һ��pH����
b��NaClO�ܵ���pH����Ҫԭ�������ڷ�����ӦClO��+ H+ HClO��ClO������H+���Ӷ��ﵽ����pH��Ŀ��
HClO��ClO������H+���Ӷ��ﵽ����pH��Ŀ��
c��NaClO�ܵ���pH����Ҫԭ��������NaClOˮ��ClO��+ H2O HClO+OH����OH������H+ ���Ӷ��ﵽ����pH��Ŀ��
HClO+OH����OH������H+ ���Ӷ��ﵽ����pH��Ŀ��
��ʵ����������������Ϊ20.0%��CuSO4��Һ�����Ƹ���Һ�����CuSO4��5H2O��H2O������֮��Ϊ ��

�ٵ缫b���� �����������ԭ������Ӧ��
��CO2�ڵ缫a�ŵ�ķ�Ӧʽ�� ��
��2����ҵ����ij����������Cu2O��Al2O3��Fe2O3��SiO2����ȡͭ�IJ����������£�

��֪�� Cu2O + 2H+ =" Cu" + Cu2+ + H2O
| ������ | Cu(OH)2 | Al(OH)3 | Fe(OH)3 | Fe(OH)2 |
| ��ʼ����pH | 5.4 | 4.0 | 1.1 | 5.8 |
| ������ȫpH | 6.7 | 5.2 | 3.2 | 8.8 |
�ٹ�������A�еijɷ��� ��
�ڷ�Ӧ����ɺ���Ԫ�صĴ�����ʽΪ ���������ӷ��ţ�
��д�����ɸ����ӵ����ӷ���ʽ ��
��x����ֵ��Χ��3.2��pH��4.0��y��Ӧ����ֵ��Χ�� ��
�����й���NaClO��pH��˵����ȷ���� ������ţ���
a������NaClO��ʹ��Һ��pH����
b��NaClO�ܵ���pH����Ҫԭ�������ڷ�����ӦClO��+ H+
 HClO��ClO������H+���Ӷ��ﵽ����pH��Ŀ��
HClO��ClO������H+���Ӷ��ﵽ����pH��Ŀ��c��NaClO�ܵ���pH����Ҫԭ��������NaClOˮ��ClO��+ H2O
 HClO+OH����OH������H+ ���Ӷ��ﵽ����pH��Ŀ��
HClO+OH����OH������H+ ���Ӷ��ﵽ����pH��Ŀ����ʵ����������������Ϊ20.0%��CuSO4��Һ�����Ƹ���Һ�����CuSO4��5H2O��H2O������֮��Ϊ ��
��1���� ������2�֣� �� CO2 + 2e- = CO+O2-��2�֣�
��2����SiO2��Cu����1�֣� ��Fe2+��2�֣� 2Fe3+ + Cu = Cu2+ + 2Fe2+��2�֣�
��5.2��pH��5.4 ��2�֣� �� b ��2�֣� �� 5��11 ��3�֣�
��2����SiO2��Cu����1�֣� ��Fe2+��2�֣� 2Fe3+ + Cu = Cu2+ + 2Fe2+��2�֣�
��5.2��pH��5.4 ��2�֣� �� b ��2�֣� �� 5��11 ��3�֣�
�����������1���ٸ��ݹ���ԭ��ʾ��ͼ��֪���缫b��O2?ת��ΪO2��ʧȥ���ӷ���������Ӧ��
��CO2�ڵ缫a�ϵõ��ӣ�ת��ΪCO��O2?���缫����ʽΪ��CO2 + 2e- = CO+O2-
��2����SiO2Ϊ����������������������Ϣ��Cu2O + 2H+ =" Cu" + Cu2+ + H2O�����Լ���ϡ�����õ��������ﺬSiO2��Cu
����Ϊ�����������е���Cu���ڣ�����FeԪ�صĴ�����ʽΪFe2+������Cu��ԭFe3+�ķ�Ӧ��2Fe3+ + Cu = Cu2+ + 2Fe2+
�ۼ���NaOH��pH=y��Ŀ������Al3+ת��ΪAl(OH)3����������y��Ӧ����ֵ��Χ��5.2��pH��5.4
��a������NaClOʹ��Һ��pH���ߣ�����b����ΪHClOΪ���ᣬ���Լ���NaClO������Ӧ��ClO��+ H+
 HClO��ʹ��ҺpH���ߣ���ȷ��c��NaClO��Һֻ������ClO?����ˮ�ⷴӦ��ClO?Ũ�ȴ� NaClO�ܵ���pH����Ҫԭ���Ƿ���������Ӧ��ClO��+ H+
HClO��ʹ��ҺpH���ߣ���ȷ��c��NaClO��Һֻ������ClO?����ˮ�ⷴӦ��ClO?Ũ�ȴ� NaClO�ܵ���pH����Ҫԭ���Ƿ���������Ӧ��ClO��+ H+ HClO������
HClO����������CuSO4?5H2O������Ϊx��H2O������Ϊy����160/250x��(x+y)��100%=20%���ɵ�x��y=5:11��

��ϰ��ϵ�д�
 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
�����Ŀ
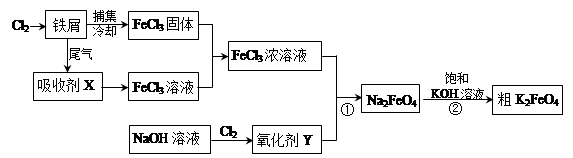
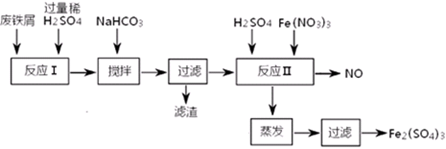
 4Fe(OH)3+8OH-+3O2�������ᴿK2FeO4ʱ�����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ�� ������ţ���
4Fe(OH)3+8OH-+3O2�������ᴿK2FeO4ʱ�����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ�� ������ţ��� CrO42-��Fe(OH)3����OH-
CrO42-��Fe(OH)3����OH-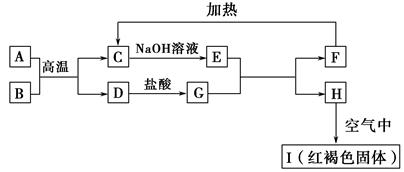
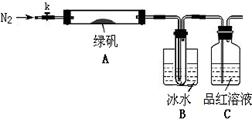
 [Cu(NH3)4]2++2OH-+4H2O,д���÷�Ӧ��ƽ�ⳣ������ʽ:������������������
[Cu(NH3)4]2++2OH-+4H2O,д���÷�Ӧ��ƽ�ⳣ������ʽ:������������������