��Ŀ����
�Ȼ���������ض��dz�����ˮ����������ͼΪ�Ʊ��Ȼ�������һ�������Ʊ�������صĹ������̡�
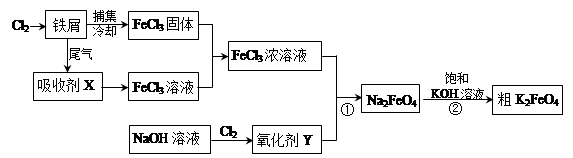
��ش��������⣺
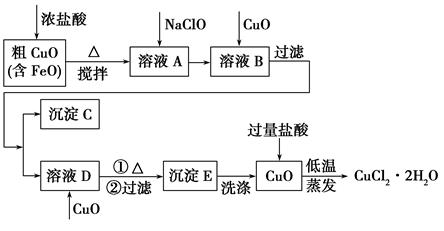
��1���Ȼ����ж�����;���������ӷ���ʽ��ʾ������;��ԭ����
���Ȼ�������ˮ��______________________��
����FeCl3��Һ��32%��35%����ʴͭӡˢ��·��____________________________��
��2�����ռ�X�Ļ�ѧʽΪ ���� ��������Y�Ļ�ѧʽΪ________________��
��3�����������·�Ӧ�ٵ����ӷ���ʽΪ____________________________________��
��4�����̢ڽ������Һ�����Сʱ�����ã����˻�ôֲ�Ʒ���÷�Ӧ�Ļ�ѧ����ʽΪ
2KOH��Na2FeO4��K2FeO4��2NaOH������ݸ��ֽⷴӦԭ��������Ӧ������ԭ��_________��
��5��K2FeO4��ˮ��Һ��������Ӧ��4FeO42-+10H2O 4Fe(OH)3+8OH-+3O2�������ᴿK2FeO4ʱ�����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ�� ������ţ���
4Fe(OH)3+8OH-+3O2�������ᴿK2FeO4ʱ�����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ�� ������ţ���
��6�����õζ��������ⶨ��K2FeO4�Ĵ��ȣ��йط�Ӧ���ӷ���ʽΪ��
��FeO42-��CrO2-��2H2O CrO42-��Fe(OH)3����OH-
CrO42-��Fe(OH)3����OH-
��2CrO42-��2H�� Cr2O72-��H2O
Cr2O72-��H2O
��Cr2O72-��6Fe2����14H�� 2Cr3����6Fe3����7H2O
2Cr3����6Fe3����7H2O
�ֳ�ȡ1��980 g�ָ��������Ʒ������������������Һ�У������Թ�����KCrO2����ַ�Ӧ����ˣ���Һ������250 mL����ƿ�С�ÿ��ȡ25��00 mL����ϡ�����ữ����0��1000 mol/L��(NH4)2Fe(SO4)2����Һ�ζ������εζ����ı���Һ��ƽ�����Ϊ18��93 mL����������Ʒ�и�����ص���������Ϊ ��
��ش��������⣺
��1���Ȼ����ж�����;���������ӷ���ʽ��ʾ������;��ԭ����
���Ȼ�������ˮ��______________________��
����FeCl3��Һ��32%��35%����ʴͭӡˢ��·��____________________________��
��2�����ռ�X�Ļ�ѧʽΪ ���� ��������Y�Ļ�ѧʽΪ________________��
��3�����������·�Ӧ�ٵ����ӷ���ʽΪ____________________________________��
��4�����̢ڽ������Һ�����Сʱ�����ã����˻�ôֲ�Ʒ���÷�Ӧ�Ļ�ѧ����ʽΪ
2KOH��Na2FeO4��K2FeO4��2NaOH������ݸ��ֽⷴӦԭ��������Ӧ������ԭ��_________��
��5��K2FeO4��ˮ��Һ��������Ӧ��4FeO42-+10H2O
 4Fe(OH)3+8OH-+3O2�������ᴿK2FeO4ʱ�����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ�� ������ţ���
4Fe(OH)3+8OH-+3O2�������ᴿK2FeO4ʱ�����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ�� ������ţ���| A��H2O | B��ϡKOH��Һ������� | C��NH4Cl��Һ������� | D��Fe(NO3)3��Һ������� |
��FeO42-��CrO2-��2H2O
 CrO42-��Fe(OH)3����OH-
CrO42-��Fe(OH)3����OH-��2CrO42-��2H��
 Cr2O72-��H2O
Cr2O72-��H2O��Cr2O72-��6Fe2����14H��
 2Cr3����6Fe3����7H2O
2Cr3����6Fe3����7H2O�ֳ�ȡ1��980 g�ָ��������Ʒ������������������Һ�У������Թ�����KCrO2����ַ�Ӧ����ˣ���Һ������250 mL����ƿ�С�ÿ��ȡ25��00 mL����ϡ�����ữ����0��1000 mol/L��(NH4)2Fe(SO4)2����Һ�ζ������εζ����ı���Һ��ƽ�����Ϊ18��93 mL����������Ʒ�и�����ص���������Ϊ ��
��1���� Fe3+ + 3H2O  Fe(OH)3(����) + 3H+��2�֣�
Fe(OH)3(����) + 3H+��2�֣�
�� 2Fe3+ + Cu��2Fe2+ + Cu2+��2�֣���2��FeCl2��NaClO����1�֣�
��3��2Fe3����3ClO����10OH����2FeO42����3Cl����5H2O��2�֣�
��4��K2FeO4�ܽ��С���������壬�ٽ���Ӧ���У�2�֣���5��B��2�֣���6��63��1%��2�֣�
 Fe(OH)3(����) + 3H+��2�֣�
Fe(OH)3(����) + 3H+��2�֣��� 2Fe3+ + Cu��2Fe2+ + Cu2+��2�֣���2��FeCl2��NaClO����1�֣�
��3��2Fe3����3ClO����10OH����2FeO42����3Cl����5H2O��2�֣�
��4��K2FeO4�ܽ��С���������壬�ٽ���Ӧ���У�2�֣���5��B��2�֣���6��63��1%��2�֣�
�����������1��������ˮ��������������������������Կ�������ˮ������ԭ���ɱ�ʾΪFe3+ + 3H2O
 Fe(OH)3(����) + 3H+��
Fe(OH)3(����) + 3H+���������Ӿ��������ԣ�����������ͭ������FeCl3��Һ��32%��35%����ʴͭӡˢ��·���ԭ���ɱ�ʾΪ2Fe3+ + Cu��2Fe2+ + Cu2+��
��2�����ռ�X������β�����������Ȼ�����Һ�����XӦ�����Ȼ���������ѧʽΪFeCl2������������������Һ��Ӧ�����Ȼ��ơ��������ƺ�ˮ�����������Ȼ������Ǵ������ƣ���ѧʽΪNaClO��
��3����Ӧ�������ô������������Ȼ����Ʊ��������ƣ����ݵ��ӵ�ʧ�غ��Լ�ԭ���غ��֪����Ӧ�����ӷ���ʽΪ2Fe3����3ClO����10OH����2FeO42����3Cl����5H2O��
��4��Ҫ�������ֽⷴӦ����������лӷ������ʻ��ѵ������ʻ����������ʣ���˸��ݷ���ʽ2KOH��Na2FeO4��K2FeO4��2NaOH��֪���÷�Ӧ֮�����ܷ���������K2FeO4�ܽ��С���������壬�ٽ���Ӧ���С�
��5�����ݷ���ʽ4FeO42-+10H2O
 4Fe(OH)3+8OH-+3O2����֪���ᴿK2FeO4Ӧ�����Ƹ÷�Ӧ��������õ��Լ���ϡKOH��Һ������ƽ�⣩�����������С�ܽ⣩����ѡB��
4Fe(OH)3+8OH-+3O2����֪���ᴿK2FeO4Ӧ�����Ƹ÷�Ӧ��������õ��Լ���ϡKOH��Һ������ƽ�⣩�����������С�ܽ⣩����ѡB����6�����ݷ�Ӧ�٢ڢۿ�֪
FeO42-����������6Fe2��
1mol 3mol
n 0��1000 mol/L��0��01893L
���n��6��31��10��4mol
��������Ʒ�и�����ص�����������
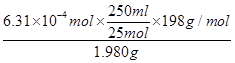 ��100%��63��1%
��100%��63��1%
��ϰ��ϵ�д�
 ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д�
�����Ŀ
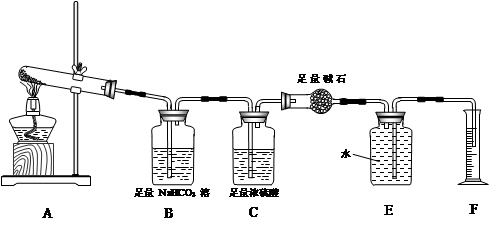 ��ʵ����̡�
��ʵ����̡�
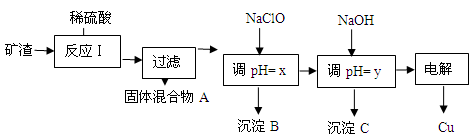
 HClO��ClO������H+���Ӷ��ﵽ����pH��Ŀ��
HClO��ClO������H+���Ӷ��ﵽ����pH��Ŀ��