��Ŀ����
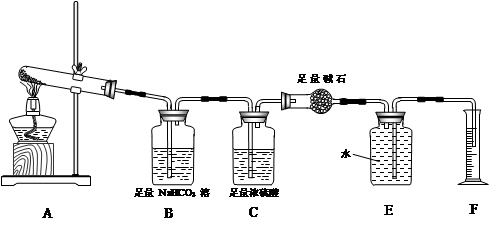
��֪A��I��Ϊ��ѧ��ѧ�еij������ʣ�����֮���ת����ϵ��ͼ��ʾ������A��DΪ�������ʣ���Ӧ��������Ҫ�����ɵ�ˮ���������ֲ�������ȥ����ش��������⣺
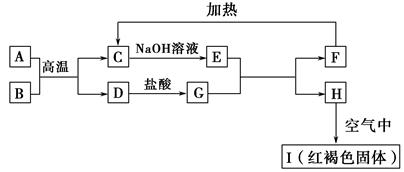
(1)B��F�ֱ��� (�ѧʽ)��
(2)д�����з�Ӧ�Ļ�ѧ����ʽ��
��A��B�ڸ����·�Ӧ�� ��
��H�ڿ�����ת��ΪI�� ��
(3)E��Һ����������Ũ���ɴ�С��˳���� ��
(4)�����ӷ�Ӧ����ʽ��ʾG��Һ�����Ե�ԭ�� ���÷�Ӧ��ƽ�ⳣ��Ϊ (��֪�����£�H���ܶȻ�����Ksp��8.0��10��16)��

(1)B��F�ֱ��� (�ѧʽ)��
(2)д�����з�Ӧ�Ļ�ѧ����ʽ��
��A��B�ڸ����·�Ӧ�� ��
��H�ڿ�����ת��ΪI�� ��
(3)E��Һ����������Ũ���ɴ�С��˳���� ��
(4)�����ӷ�Ӧ����ʽ��ʾG��Һ�����Ե�ԭ�� ���÷�Ӧ��ƽ�ⳣ��Ϊ (��֪�����£�H���ܶȻ�����Ksp��8.0��10��16)��
(1)Fe2O3��Al(OH)3
(2)��Fe2O3��2Al 2Fe��Al2O3
2Fe��Al2O3
��4Fe(OH)2��2H2O��O2=4Fe(OH)3
(3)c(Na��)��c([AlCO4]��)��c(OH��)��c(H��)
(4)Fe2����2H2O Fe(OH)2��2H�� 1.25��10��13
Fe(OH)2��2H�� 1.25��10��13
(2)��Fe2O3��2Al
 2Fe��Al2O3
2Fe��Al2O3��4Fe(OH)2��2H2O��O2=4Fe(OH)3
(3)c(Na��)��c([AlCO4]��)��c(OH��)��c(H��)
(4)Fe2����2H2O
 Fe(OH)2��2H�� 1.25��10��13
Fe(OH)2��2H�� 1.25��10��13����������ͼΪ���壬������������ʼ���ѧ��Ӧԭ�������ڿ��鿼�����ۺϷ���������(1)���ݿ���ת����ϵ�������Ϣ������ȷ��IΪ����������A��DΪ�������ʣ�����A��B�ķ�Ӧ�����Ϳ������뵽���ȷ�Ӧ���Ʋ�A��DΪ����������AΪ����BΪ��������CΪ��������DΪ����EΪƫ�����ƣ�GΪ�Ȼ�������FΪ����������HΪ������������IΪ����������ƫ������ˮ��ʹ��Һ�ʼ��ԣ�������Һ������Ũ�ȵĴ�С˳��Ϊc(Na��)��c(AlO2-)��c(OH��)��c(H��)��FeCl2��Һ�У�Fe2������ˮ�⣺Fe2����2H2O Fe(OH)2��2H����ƽ�ⳣ��K��
Fe(OH)2��2H����ƽ�ⳣ��K��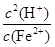 ��
��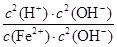 ��
��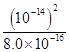 ��1.25��10��13��
��1.25��10��13��
 Fe(OH)2��2H����ƽ�ⳣ��K��
Fe(OH)2��2H����ƽ�ⳣ��K��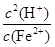 ��
��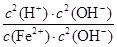 ��
��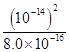 ��1.25��10��13��
��1.25��10��13��
��ϰ��ϵ�д�
�����Ŀ

 ��ʵ����̡�
��ʵ����̡�
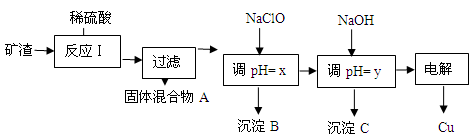
 HClO��ClO������H+���Ӷ��ﵽ����pH��Ŀ��
HClO��ClO������H+���Ӷ��ﵽ����pH��Ŀ��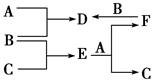
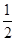 (a-b)mol
(a-b)mol